Cleaning is intended to remove soils (e.g., patient secretion, tissues and inorganic material such as salts) from surfaces of reusable medical devices (RMD). Although cleaning takes place after point of use processing, patient secretions, tissues and inorganic material such as salt may remain on RMD surfaces. They could interfere with sterilizing or disinfecting agent or generate endotoxin or pyrogen risks
Disinfection, is intended to reduce the microbial load. Disinfection may be the last step before use of an RMD or take place in preparation for sterilization.
- Before sterilization, disinfection improves the preparation of a RMD for sterilization. It may be required or recommended in some countries as an occupational health and safety measure for operators in charge of packaging.
- As the last step before use of an RMD, the objective of disinfection is to render the RMD safe for the patient according to Spaulding classification principles.
![]() Although cleaning and disinfection are different in practice, they are often grouped in a cleaning and disinfection process (e.g., automated washer disinfector). Therefore they were grouped together in a single chapter of these guidelines
Although cleaning and disinfection are different in practice, they are often grouped in a cleaning and disinfection process (e.g., automated washer disinfector). Therefore they were grouped together in a single chapter of these guidelines
Cleaning consists of washing followed by rinsing
- Washing is using water that contains a cleaning agent to remove soils from RMD surfaces.
- Rinsing clears the soils removed by washing as well as cleaning agent residues which would chemically interact with disinfection or sterilization agents.
Main categories for detergent are: (1) neutral with or without enzymes, (2) mild alkaline with or without enzymes, (3) alkaline
Most detergent formulations include a surfactant, which reduces the surface tension of water thus easing the wetting of surface and breaking up of soils). Other components of detergents are buffers which improve the compatibility with RMD material and softeners which reduce the potential negative effect of hard water (spotting and deposit on surface).
|
Cleaning is performed according to the manufacturers’s IFU’s (instructions for use) of an RMD, cleaning equipment and cleaning agents.
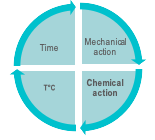
The 4 conditions for efficient washing are illustrated by the Sinner circle. 1. Mechanical action by manual brushing, flushing, swabbing or wiping, automated spraying or flushing, cavitation (in ultrasonic cleaner). 2. Chemical action of cleaning agents that decompose protein, lipids and ease the removal of soils from surfaces 3. Contact time of all RMD surfaces with the cleaning agent and water solution. 4. Temperature of the cleaning solution. Concentration, exposure time or temperature exceeding the instructions of the cleaning agent manufacturer may present a risk for operators, damage an RMD, and do not mean greater cleaning efficacy.
|  |
Consistency of cleaning (i.e. regular application of a cleaning procedure after each use of an RMD) is key to avoid the progressive formation of biofilm or build-up of mineral deposits in narrow spaces or cavities
| Biofilms are a biomass of bacteria and extracellular material. Biofilms (e.g., biofilms of Pseudomonas aeruginosa) tightly adhere to surfaces of an RMD and can protect the germs from exposure to the disinfection agent. Biofilms grow over time and become difficult to remove, even if subsquent cleanings are thoroughly executed. Build-up of a mineral deposit (e.g., scale) can aid attachment of bacteria to an RMD surface, allowing biofilm formation. Deposit may also damage instruments and impair their function. Scratches or rust facilitate the build-up of soils and biofilms. Damaged or rusted RMD's are sent for maintenance or discarded. |
Disinfection is chemical or thermal
- Chemical disinfection with a disinfectant or cleaning and disinfectant formulation is followed by rinsing, drying and when recommended by an RMD IFU, by lubrication.
Rinsing with adequate water quality (water hardness, pH and temperature) removes chemical agent residues. Rinsing after disinfection is often called final rinsing to differentiate it from intermediary rinsing which takes place after cleaning. Drying with adequate air quality removes residual humidity which favors recontamination and impairs sterilization. In some countries, alcohol rinsing is used to accelerate drying (specifically for GI endoscope reprocessing). This practice is not allowed in Europe and some other countries due to fixative properties of alcohol.
Lubrication is implemented when specified in an RMD manufacturer's IFU. Lubricants are intended to protect against friction on the surfaces of an RMD. RMD manufacturer's IFU's specify the type of lubricant to be used and which parts of an RMD requiring lubrication. Non-approved lubricants can interfere with sterilization or disinfection, create harmful by-products, and/or damage the device or the sterilizer/disinfection equipment. Water soluble lubricants are commonly preferred. |
Chemical disinfection is used for thermosensitive RMD’s. An RMD is immersed in a bath (using a manual process), exposed to spray (using an automated process) or wiped (used only when immersion or spray are not allowed according to an RMD manufacturer’s IFU).
Chemical disinfection is achieved when all RMD surfaces have been exposed to the disinfecting formulation at concentration, temperature and for the contact time specified by the disinfectant manufacturer’s IFU.
Disinfection is characterized by the achievement of a specified log10 reduction of representative test microorganisms.
Categories of microorganisms are listed below in order of usual growing order of resistance to disinfectant (examples of microorganisms frequently used for tests appear in brackets)
Prions are more resistant than all the above. Each claim (bactericidal, fungicidal, virucidal, mycobactericidal and sporicidal) is validated separately. For example, a successful sporicidal test, does not yield a claim with germs of lower resistance. Tests microrganisms are protected by artificial soil simulating the level of organic matter which may be found on RMD. Disinfectant used after cleaning is tested in clean conditions. Disinfectant used for combined cleaning and disinfection are tested in dirty conditions. Disinfectant must comply to regionally applicable international standards. There are no unique international standards for evaluation of disinfecting activities. Depending on the region applicable standards are, for example, AOAC, ASTM or EN. |
Chemical disinfectants formulations may contain oxidizers (hydrogen peroxide, peracetic acid), quaternary ammonium compounds, chloride or aldehydes (Glutaraldehyde, Orthophtalaldehyde). Detergent-disinfectants use chemical with dual properties or mixtures of detergent and disinfectant .
|
Room ventilation is required for glutaraldehyde due to irritancy and potential toxicity. Orthophtalaldehyde (OPA) is better tolerated although ventilation is also required Aldehydes are fixative of protein and must be only be used after thorough cleaning. Peracetic acid has a broad spectrum of activity and good efficacy on spores, but is less stable and can more corrosive than aldehydes depending on formulations. Unlike aldehydes it produces no toxic waste. Other formulations based on hydrogen peroxide, chlorine based, chlorine dioxide (ClO2) may be found. Compatibility with materials of RMD's must be checked. Alcohol is not an effective disinfectant for an RMD (it does not penetrate well into organic material and is not sporicidal) . Combined cleaning and disinfecting chemical formulations are mixtures of quaternary ammonium, biguanide, guanidine, alkylamine, enzymatic or alkaline. Aldehyde-based cleaner-disinfectants are avoided due to their fixative properties. |
Choice of disinfectant involves a partnership with infection control. Disinfectant must be effective on the type and quantity of microorganisms that may be present on an RMD.
A manufacturer of a chemical washer-disinfector, defined periodicity and a method for self-disinfection of the chemical WD. The objective of self-disinfection is to eliminate contaminants that may have accumulated over time within the WD. Self-disinfection can be thermal (i.e., hot water) or chemical. If chemical, local guidelines may require a disinfectant different from the one used for reprocessing cycles.
For instance, the type and amount of microorganisms present on an RMD are not the same for gastroscopes and bronchoscopes. |
- Thermal disinfection is performed in automated washer-disinfectors (WD) with hot water at specified temperature. Thermal disinfection is commonly used for surgical instruments intended for steam sterilization. Lubrication is applied as recommended by RMD manufacturer IFU.
Reusable containers and other heat and moisture compatible items are also typically thermally disinfected. WD disinfection and rinsing are combined in thermal disinfection. Thermal disinfection provides good drying. A rinse aid may be added to the rinse water. Thermal disinfection is also efficient for self-disinfection of the washer-disinfector.
![]() Manufacturers of thermal WD may however offer or recommend periodic self-disinfection cycles
Manufacturers of thermal WD may however offer or recommend periodic self-disinfection cycles
Thermal disinfection, is achieved when all RMD surfaces have been exposed to hot water at a defined temperature for a minimum contact time.
Thermal disinfection is efficient to eliminate most microorganisms. Spores show higher resistance. Thermal disinfection can be characterized by the A0 concept.
The improvement of lethality with hot water temperature can be predicted. For instance, for heat resistant microorganisms, 10 minutes at 80°C has the same lethality effect as 1 minute at 90°C or 100 minutes at 70°C. By convention the time (expressed in second) to obtain a given log reduction at 80°C serves as a reference and called A0 for heat resistance microorganisms. In the previous example A0 equals 600 s (10 minutes at 80°C). Higher A0 means longer exposure time at 80°C or shorter time at higher temperature according to the A0 equation.
See annex B of international standard ISO 15883-1 for more information. Local regulation, recommendations or habits define the A0 thresholds according to the level of contamination of an RMD and its intended use. A0 of 60 s is commonly recognized as the minimum to be targeted for low risk items according to Spaulding classification principles. 600 s is the minimum for other items. Automated WD for surgical instruments complying to ISO 15883-2 must have one cycle with an A0 value above 3000 (e.g. a value of 50 minutes at 80°C or practically 5 minutes at 90 °C or 2 min and 30 s at 93 °C). A0 values of 3000 or above are recommended in some countries. Others consider 600 s as sufficient for most items (considering that thermal disinfection always takes place after cleaning and that residual spores (if any) will be eradicated by sterilization
|
Even after disinfection, an RMD may still carry some microorganisms and residual humidity and there are not protected by packaging. Precaution must be taken to limit environmental and handling contamination.
Maximum storage time after disinfection are usually defined by local guidelines or regulations.
The 4 main categories of cleaning and disinfection processes are
- Manual cleaning and disinfection. RMD’s are immersed and manually processed in cleaning and then disinfection baths, or in combined cleaning and disinfection solutions. When immersion is not allowed according to an RMD manufacturer’s IFU, an RMD is wiped off.
- Automated washer disinfector (for surgery and dentistry RMD’s). Disinfection is thermal or chemical
- Ultrasonic cleaning, when approved, according to an RMD manufacturer IFU,
- Automated Endoscope Reprocessors (AER) for thermosensitive flexible endoscopes
The Choice of cleaning and disinfection process, cleaning, disinfection and lubrication agents go by the RMD manufacturer’s IFU, and detergent as well as cleaning equipment manufacturers IFU’s.
Tests are performed with microorganisms known for their high resistance to the sterilization process (usually bacterial spores).
Once the process has been characterized, it must be verified that an RMD can be efficiently sterilized. The 106 inoculum is placed at the position determined as the most difficult to sterilize on or within an RMD. RMD's are packaged and inserted in a representative challenging load. For routine controls, inoculums may be placed in process challenge devices (PCD) |  |
When allowed according to the IFU of an RMD manufacturer IFU, automated cleaning and disinfection processes (WD and AER) are preferred over manual processes.
Washer-disinfector (WD) and automated endoscope reprocessors (AER) are more reproducible (less human dependent), safer for operators (reduction of exposure to chemicals, aerosols, handling risk) and provide automatic recording.
For the cleaning and disinfection of RMD in preparation for steam sterilization International standards express a preference for thermal disinfection
![]() ISO 15883-12 says: Thermal disinfection processes are more easily controlled and avoid the hazards to staff, patients and the environment that can occur through the use of chemical disinfectants. “Thermal disinfection is efficient to eliminate most microorganisms (except spores) and provide good rinsing and drying”.
ISO 15883-12 says: Thermal disinfection processes are more easily controlled and avoid the hazards to staff, patients and the environment that can occur through the use of chemical disinfectants. “Thermal disinfection is efficient to eliminate most microorganisms (except spores) and provide good rinsing and drying”.
Manual or ultrasonic pre-cleaning may be required before automated cleaning and disinfection of complex and/or heavily soiled RMD.
Manual wiping is used when immersion in bath is not allowed by RMD manufacturer IFU
![]() International standard ISO 176648says :
International standard ISO 176648says :
At least one validated automated cleaning method (which may include a validated manual pre-cleaning method) shall be specified unless the medical device cannot withstand any such process, in which case a statement shall be provided which alerts the user to this issue. A validated method of manual cleaning shall be specified if automated cleaning is not possible.
if the medical device is intended to be disinfected, at least one validated automated disinfection method shall be specified unless the medical device cannot withstand any such process, in which case a statement shall be provided which alerts the user to this issue. If the medical device is intended to be disinfected, a validated method of manual disinfection shall be specified if automated disinfection is not possible
Manual Cleaning and disinfection processes are prepared and implemented according to written standard operating procedures (SOP’s).
Preparation of cleaning and disinfection baths is done according to the IFU of the cleaning agent manufacturer. Water quality used for each step is as recommended per IFU (water hardness, pH, temperature).
Brushes, single-use cloth and other cleaning accessories are as recommended by RMD Manufacturer’s IFU or equivalent brushes (usually soft nylon bristle brushes). Unless clearly specified in an RMD IFU, metallic brushes are not used. Type, size (diameter and length), bristle type and material of brushes used to clean lumen are those indicated in an RMD manufacturer’s IFU. They should be the same diameter as the lumen to ensure all internal surfaces can be reached and long enough to exit the distal end of the instrument. RMD manufacturers may have special recommendation for optics surfaces.
If not single use, brushes are cleaned daily, disinfected (preferably by thermal process) and dried. Brushes are checked after each use and replaced when damaged.
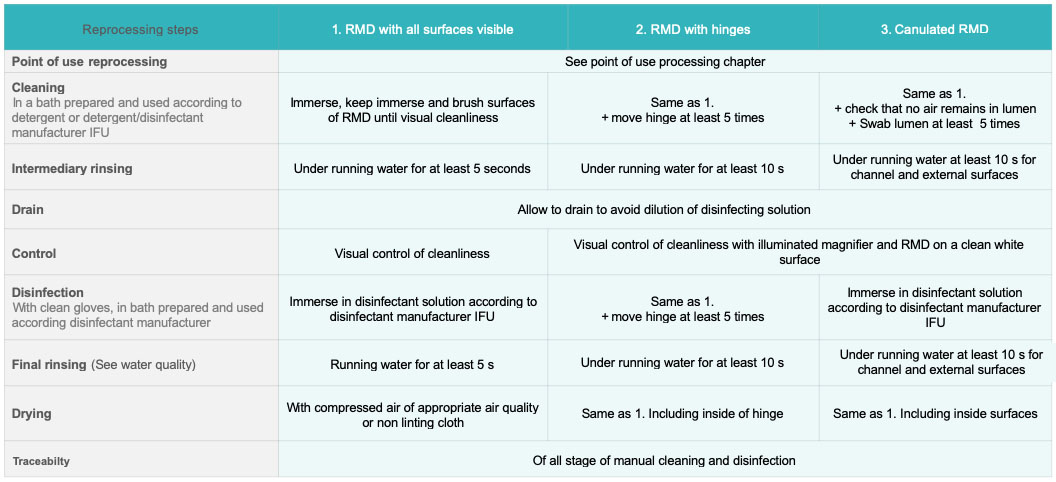
After disassembly according to RMD manufacturer IFU, if needed, steps are as follows:
- The RMD is completely immersed in the cleaning bath. Articulated RMD are open to minimize obscured surface area. Bubbles trapped in cavities and channels are eliminated. Some manufacturers may recommend flushing through at a specified pressure for a defined time. RMD remains immersed during cleaning. Cleaning operations are performed in a manner that limits production of aerosols or dispersion of potential contaminated chemicals or water.
- Gross soil is removed. Accessible surfaces are cleaned with appropriate tools until visibly cleaned. RMD’s with articulations are moved (open and close at least 5 times). Channels are brushed. Brushing is always performed in the same direction from the less soiled to the most soiled end (at least 5 times). Other hard to access area are flushed with syringe, water gun or hand pump
- An RMD is rinsed under running water (at least for 10 s for all external surfaces). Articulations are moved. Channels are flushed for at least 10 s. An RMD is allowed to drain to avoid dilution of disinfection solution. Rinsing removes visible and non-visible soil as well as residual detergent which might react with disinfectant.
- Absence of soil is visually checked. An RMD and cannulated device are placed on a white clean surface (for instance white crepe papier) and observed under illuminated magnifier
- An RMD is immersed in a disinfection bath. Articulations are moved at least 5 times. It must be checked that internal lumen and cavities are completely perfused and in contact with solution.
- An RMD is rinsed with appropriate water quality: 10 s at least for all external surface and 10 s flush for each channel.
- An RMD is dried with adequate compressed air quality. If compressed air is not available or not recommended by the manufacturer’s IFU, an RMD is air-dried (with medical compressed air) or hand-dried with a disposable clean, lint-free cloth.
If manual cleaning and disinfection is performed with a cleaning-disinfecting agent steps 2 and 3 are skipped and visual control is performed after 6.
The disinfection bath is preferably single use. If not, the disinfectant manufacturer should specify the maximum number of reuse acceptable and provide recommendations and means to control that the concentration of disinfection agent is above minimum levels required for efficacy (for example, test strips).
SOP’s details the steps and, for instance, provide quantitative and qualitative indication for manual operation, number of times a channel should be brushed or flushed and accessories to be used
It may be useful to organize an SOP regarding Manual Cleaning and Disinfection according to an RMD configuration.
 |
For an RMD or parts of an RMD that cannot be immersed (e.g., some powered instruments or batteries) wipe and rinse with disposable cloth soaked with detergent, disinfectant agents, or both. Consistency of manual wiping is difficult to obtain. Manual wiping is used when other cleaning and disinfection methods are not applicable.
Manual wiping is performed according to an RMD manufacturer IFU. :
- Wipe the RMD with a disposable, clean, lint-free cloth until all soil is visually removed. Check that moisture does not penetrate into critical parts of the device (for instance power or electronic connections)
- Rinse the RMD by wiping the surface thoroughly with a damp, disposable, clean, lint-free cloth with the objective of withdrawing disinfection residues.
- If needed, visually control the RMD with an illuminated magnifier
- Dry the RMD with medical-grade compressed air. If compressed air is not available or not recommended by the manufacturer of the RMD, air dry or hand-dry using a disposable clean, lint-free cloth. Discard disposable cloths.
If wiping is performed with a cleaning and disinfecting agent (non-critical RMD only) steps 2 and 3 are disregarded.
Ultrasonic cleaners use cavitation to detach soils from an RMD.
Ultrasound generators produce acoustic waves (waves frequency generally ranges between 35 and 45 kHz). Acoustic waves create bubbles which implode as they become larger and unstable. Implosion creates a vacuum in the solution (cavitation) that mechanically draws debris from the surface of the RMD. Ultrasonic cleaners encompass a wide range of technologies from the basic ultrasonic cleaning equipment with the user performing all the function of filling, rinsing, and draining to automated equipment. Ultrasonic cleaners could be categorized as follows : (1) (basic) ultrasonic cleaners, (2) ultrasonic irrigators, (3) irrigator-washer, and (4) irrigator washer-disinfector. |
- Efficacy of cavitation is improved by adapted cleaning agent.
- Ultrasonic cleaning is used only if it is not contraindicated by an RMD IFU.
- Ultrasonic cleaners are often used for pre-cleaning (prior to Washer-disinfector) of a complex RMD. They are well-adapted for stainless steel devices with reamers (rasps) , lumen, sleeves or complex shapes that are difficult to access.
Ultrasonic cleaning is not recommended for an RMD with :
|
- Ultrasonic cleaning processes are prepared and implemented according to a written standard operating procedure (SOP) adapted to ultrasonic cleaner features.
- Detergent must be suitable for ultrasonic cleaner.
- Before immersion in an ultrasonic bath RMD gross soil is removed.
Principles for bath preparation, loading and unloading are as follows
- Follow the instructions of ultrasonic cleaner and detergent manufacturers for dosing and temperature of bath. The water temperature is between 27 °C (80 F) and 43 °C (109 F) and never above 60 °C (140 F) because most proteins denaturate above that temperature. For ultrasonic systems without thermoregulation room temperature might be preferable as energy delivered by the ultrasonics wave will increase the temperature of the bath.
- The bath is degassed at each filling. This is done by filling the unit, closing the lid and running a cycle for 5 to 10 minutes
- RDM are placed in a tray (never directly on the tank end) and open wide, not superimposed to avoid shadowing. When applicable, connect lumen devices to flushing ports. If other specific positioning accessories and features are used, refer to the manufacturer’s IFU.
- The cavitation process may create aerosols, so the ultrasonic bath should have a lid that must remain closed during the cleaning process.
- The process time is per the manufacturer recommendations (usually between 5 and 10 minutes)
- If rinsing is not performed by the ultrasonic cleaner, the RMD is rinsed and dried manually
The bath is preferably changed after each use (i.e. after reprocessing cycle). Ultrasonic cleaning equipment is cleaned every day that it is used and according to manufacturer IFU. Local recommendations specify that it has to be refilled after each use or daily and each time the bath is visibly soiled.
![]() National regulation or recommendations may forbid the use of ultrasonic cleaners if a prion risk has been identifiedMD
National regulation or recommendations may forbid the use of ultrasonic cleaners if a prion risk has been identifiedMD
Washer-Disinfectors spray or flush pressurized water mixed with a detergent on surfaces of RMD and lumen. The cleaning stage is followed by a thermal or chemical disinfection.
Various types of WD are:
- Washer-disinfectors with a thermal or chemical disinfection stage for surgical instruments; these are available in different configurations, e.g. single chamber (pass-through or single door) or multi-chamber versions.
- Cart washers for carts, reusable containers, surgical basins and other non-invasive , non-critical medical devices; these can be single or multi chamber (steps of the cleaning and disinfection process are performed in different chambers).
- A table top washer-disinfector for dental handpieces and turbines.
![]() Automated endoscope reprocessor AER are described separately in automated endoscope reprocess (AER) paragraph
Automated endoscope reprocessor AER are described separately in automated endoscope reprocess (AER) paragraph
Except for table top washer-disinfector for dental handpieces, WD’s are covered by international standards
![]() International Standard ISO 15883 includes general requirements ISO 15883-1 and specific parts dedicated to each type of WD;
International Standard ISO 15883 includes general requirements ISO 15883-1 and specific parts dedicated to each type of WD;
- WD for instruments comply with ISO 15883-1 and -21,2
- Cart washer comply with ISO 15883-1 and -6 (thermal disinfection) or -7 (chemical disinfection)1,6,7
There is currently no international standard for table-top dental handpieces and turbines washer-disinfector. Quality and performances of a table-top washer-disinfector significantly vary. Some washer-disinfectors that comply with international standards can be equipped with dental adaptors.
1![]() Compliance to ISO 15883-11 and subsequent parts2,3,4,5,6,7 may be required (for instance in Western Europe)
Compliance to ISO 15883-11 and subsequent parts2,3,4,5,6,7 may be required (for instance in Western Europe)
WD processes are prepared and implemented according to written standard operating procedure (SOP).
RMD’s that are heavily soiled, complex, or both may require manual or ultrasonic precleaning
When applicable, disassembly of a multicomponent RMD is performed according to an RMD manufacturer’s IFU. Cap, gaskets and all disposable items are removed. Hinged surgical instrument with handles (scissors, clamp, and forceps) are opened (generally at 90°) according to the manufacturer’s IFU’s.
An SOP should describe the load configuration (i.e., the type of RMD’s that can be reprocessed together in a given cycle).
RMD’s are placed in a tray or positioned on suitable modules and/or connected to irrigation ports (for lumen devices).
If needed using hold-down screens or retaining systems prevent dislodging of an RMD during the cycle. Fragile or small devices are protected (installed in trays with lids, blocking accessories, or both). Heavy or large items are preferably placed on lower-levels Hollow items are turned downwards for water evacuation Racks should never be overloaded. All RMD surfaces must be exposed to water spray (no shadowed surface). Specialized racks and loading equipment are required for RMD's with complex geometries and lumen. Racks are for instance available for laparoscopic surgery, dentistry, robotic surgery. Minimally Invasive Surgery (MIS) instruments with lumen are connected to adaptors. Adaptors are carefully chosen for proper flushing pressure and flow. Clear procedures are key to optimize the layout; avoid connection mistakes or accidental disconnection during cycles. In case of particularly narrow lumina a filter is required to prevent particles from entering the lumen. |
Checked that spraying arms are unobstructed before cycle launch.
All phases of WD cycles are automatically run, controlled and recorded. WD cycles usually start with a pre-washing phase (included in the automated cycle) to moisten an RDM, but this does not replace ultrasonic or manual pre-washing.
- Pre-washing moistens the RMD (without detergent) to improve soil removal.
- Washing is done at the temperature specified by a detergent manufacturer (usually around 45 °C. Mechanical action is by spray, circulation or irrigation). The cleaning solution is single use.
- Intermediary rinsing may include a neutralization stage (for some cleaning agent formulations)
- Thermal (preferred if applicable) or chemical disinfection is adapted to contaminants and log reduction targets. In chemical WD, if the disinfectant is not single use, the maximum number of reuse specified by the disinfectant manufacture is followed. Recommendations and means are provided by the manufacturer to control that the concentration of disinfection agent is above minimum levels required for efficacy.
- Final Rinsing. In thermal WD thermal disinfection also serves as a rinsing phase.
- Drying is obtained by circulation of hot air (of appropriate air quality).
Automated endoscope reprocessors are used for gastro-intestinal (GI) scopes (GI) and some other semi-critical single lumen flexible endoscopes (e.g., bronchoscopes, ureteroscopes). AER spray or flush pressurized water is mixed with a detergent and then spray or flush a disinfectant on surfaces and lumens of the endoscopes. All phases are automatically run, controlled and recorded.
Written cleaning and disinfection standard operating procedures (SOP) are prepared in accordance with quality management principles. Each step of the cleaning& disinfection process is an improvement to the former steps and does not impair the efficacy of the subsequent stages.
For instance, final rinsing and drying are performed with adapted water quality and air quality to avoid recontamination of a disinfected RMD.
User supervises or performs process validation and, in particular, controls that:
- Installation of cleaning and disinfection workstation or equipment conforms to manufacturer recommendations
- Instruction for use, maintenance manuals, and test and calibration certificates, are available
- Standard Operating Procedures (SOP’s) are up to date. SOP’s are available for each RMD or group of RMD’s requiring a similar cleaning and disinfection process. For a newly purchased RMD, a new SOP is defined if an existing one cannot be used. When possible SOP’s provide quantitative and qualitative criteria for manual operations (e.g. brush until no soil is visible, the number of times a lumen should be swabbed etc..)
- Systematic and periodic routine controls are in place (see below)
| For all cleaning and disinfection processes, RMD's are visually controlled for cleanliness. If soils are visible RMD's are cleaned again. For washer disinfector (WD) and automatic endoscope reprocessors (AERs) processes complying to international standard ISO 15883-1, cleaning efficacy is controlled periodically and according to methods described in ISO 15883-1 and 15883-55. For WDs and AERs not aiming compliance to ISO 15883 validation framework proposed by ISO 15883 and 15883-5 methods may be used Microbiological and chemical quality of incoming and final rinsing water are controlled according to local regulation and they meet specifications of equipment, detergent and disinfectant manufacturers' IFU's. Periodical Microbiological controls of an RMD may be required by local regulations (e.g., annual control of flexible endoscopes in some countries). |
- Occupational health and safety considerations (in particular exposure to liquid and vaporized chemicals, aerosols and injuries by potentially contaminated RMD’s)
- Reprocessing fluids are discarded according to local waste management rules.
- Training (including training on occupational health and safety measures) is up to date, executed and training certificates are available.
- Maintenance plans are in place for washer-disinfector, ultrasonic cleaners and dosing pumps.
- Traceability is operational

WFHSS key recommendations for cleaning & disinfection
- The cleaning & disinfection process complies to instructions for reprocessing of an RMD manufacturer. It is implemented according to manufacturer’ IFU’s for an RMD, detergent, disinfectant and reprocessing equipement.
- Thorough and consistent Cleaning is essential for efficient disinfection and sterilization. Progress of minimally invasive surgery often means complex, narrow geometries, difficult to access and hidden to visual control. Inconsistent cleaning allows the progressive development of biofilms or mineral deposits.
- Objective of disinfection depends on intended use of RMD
-
- When done in preparation for sterilization, disinfection improves the preparation of RMD for sterilization. It may be required or recommended in some countries as an occupational health and safety measure.
- When disinfection is the last step before use of the RMD on a patient, targeted efficacy is defined, with infection control, according to Spaulding classification principles. Disinfectant must comply to locally applicable international standards
- Automated cleaning and disinfection in a WD or an AER is preferred to manual
-
- Ultrasonic or manual precleaning may be needed for complex or heavily soiled RMD’s. Ultrasonic reprocessing must be allowed by an RMD manufacturer’s IFU.
- Thermal WD are preferred for heat and moisture resistant RMD.
- WD and AERs that comply with international standards1,2,3,4,5,6,7are preferred
- Quality and performance of Dentistry washer-disinfector must be controlled
- Non automated cleaning and disinfection is performed with care and consistency. When specified by RMD manufacturer’s IFU, ultrasonic cleaning is efficient for devices with complex geometries. Manual wiping is used only when an RMD manufacturer’s IFU does not allow immersion. Combined manual cleaning and disinfection with a cleaning&disinfecting formulation may be used on low risk items according to Spaulding classification principles or, if permitted by local regulation, in preparation for sterilization.
- Manual and automated cleaning and disinfection processes are implemented according to quality management principles. Standard operating procedures (SOP) are up to date and describe systematic or periodic (visual controls of cleanliness and dryness are systematic). Process validation concerns both automated and manual processes. Operators training is regularly updated and controlled. Appropriate occupational health and safety measures and traceability are in place.
Cleaning & disinfection before use or in preparation for sterilization
Go to Objective of cleaning & disinfection →
1 of 12 Clean, dry, disinfected RMDRMD ready for use or ready for sterilization
Go to Objective of cleaning & disinfection →
2 of 12 PrecleaningManual precleaning for flexible scope
Go to Automated endoscope reprocessing (AER) →
Ultrasonic cleaning if allowed by RMD manufacturer IFU
Go to Ultrasonic cleaning →
AER for flexible GI scopes
Go to Automated endoscope reprocessing (AER) →
WD for surgical instruments
Go to Washer Disinfector (WD) →
4 of 12 Manual cleaning and disinfectionWith cleaning and disinfection solution
Go to manual cleaning and disinfection →
5 of 12 Manual disinfectionWith disinfection solution
Go to manual cleaning&disinfection →
6 of 12 Manual cleaningWith cleaning & disinfection solution
Go to manual cleaning&disinfection →
7 of 12Automated processes unless not allowed by RMD manufacturer IFU
Validated cleaning & disinfection process
Go to ![]() WFHSS recommendations for cleaning and disinfection →
WFHSS recommendations for cleaning and disinfection →
As needed, precleaning for complex and heavily soiled RMD and for flexible scopes
9 of 12RMD visibly clean and dry
Control of process parameters for AER and WD
Go to cleaning & disinfection and quality →
10 of 12According to SOP combined or
sequential cleaning & disinfection
Go to manual cleaning&disinfection →
11 of 12Thorough and consisten cleaning,
repeat if visible soil
Go to ![]() WFHSS recommendations for cleaning & disinfection →
WFHSS recommendations for cleaning & disinfection →
- ISO 15883-1 : Washer-disinfectors — Part 1: General requirements, terms and definitions and tests (2006)
- ISO 15883-2 : Washer-disinfectors — Part 2: Requirements and tests for washer-disinfectors employing thermal disinfection for surgical instruments, anaesthetic equipment, bowls, dishes, receivers, utensils, glassware, etc. (2006)
- ISO 15883-3 : Washer-disinfectors — Part 3: Requirements and tests for washer-disinfectors employing thermal disinfection for human waste containers (2006)
- ISO 15883-4 : Washer-disinfectors — Part 4: Requirements and tests for washer-disinfectors employing chemical disinfection for thermolabile endoscopes (2018)
- ISO 15883-5 : Washer-disinfectors — Part 5: Performance requirements and test method criteria for demonstrating cleaning efficacy (new version to be published in 2020)
- ISO 15883-6 : Washer-disinfectors — Part 6: Requirements and tests for washer-disinfectors employing thermal disinfection for non-invasive, non-critical medical devices and healthcare equipment (2011)
- ISO 15883-7 : Washer-disinfectors — Part 7: Requirements and tests for washer-disinfectors employing chemical disinfection for non-invasive, non-critical thermolabile medical devices and healthcare equipment (2016)
- ISO 17664 : Processing of healthcare products — Information to be provided by the medical device manufacturer for the processing of medical devices













