![]() 朊毒体不是微生物,比普通的微生物更具抗力。
朊毒体不是微生物,比普通的微生物更具抗力。
可重复使用的医疗器械(RMD)的灭菌基于 3 个关键概念
- 无菌保证水平 (SAL):不可能系统地控制完全没有存活微生物。灭菌的目标是将微生物存活的可能性限制在一个非常低的水平。对于 RMD,该可能性或无菌保证水平 (SAL) 表示为:在 100 万个已灭菌物品中不超过有 1 个存活微生物(或 10-6 SAL)。
- 过度杀灭:可能存在于清洁后的 RMD 上的微生物的数量、性质和位置不能明确。 RMD 灭菌过程必须证明其能够灭活超过 100 万数量级的测试微生物的高浓度接种物。选择测试微生物是基于它们对灭菌过程具有高抗力。这种高于实际污染最高水平的保守裕量被称为过度杀灭法。
- 兼容性:RMD 在灭菌后能保持完整的功能和安全使用。兼容性由 RMD 制造商检查,该制造商决定了 RMD 在被丢弃或维修之前,可以暴露的最多的灭菌周期数。
国际标准提出了多种方式来证明给定灭菌过程应用过度杀灭方法达到 SAL 的能力。 其中之一是半周期过度杀灭方式,描述如下:
使用已知对灭菌过程具有高抗力的微生物(通常是细菌芽孢)进行测试。
灭活动力学是线性的,则杀灭 106 微生物的时间或剂量加倍就会产生 10-6 SAL。例如,D 值为 2 分钟的灭菌过程在 12 分钟内灭活 6 log,则在 24 分钟内可达到 SAL。 一旦对过程进行了表征,就必须验证 是否可以有效灭菌RMD。将 106 接种物放置在 RMD 上或内部最难灭菌的位置。 将包装好的RMD放入具有代表性的具有挑战性的负载中。 RMD 制造商的 IFU 指明废置或维修前的最多使用次数。 用于常规控制时,接种物可以放置在过程挑战装置 (PCD) 中 | 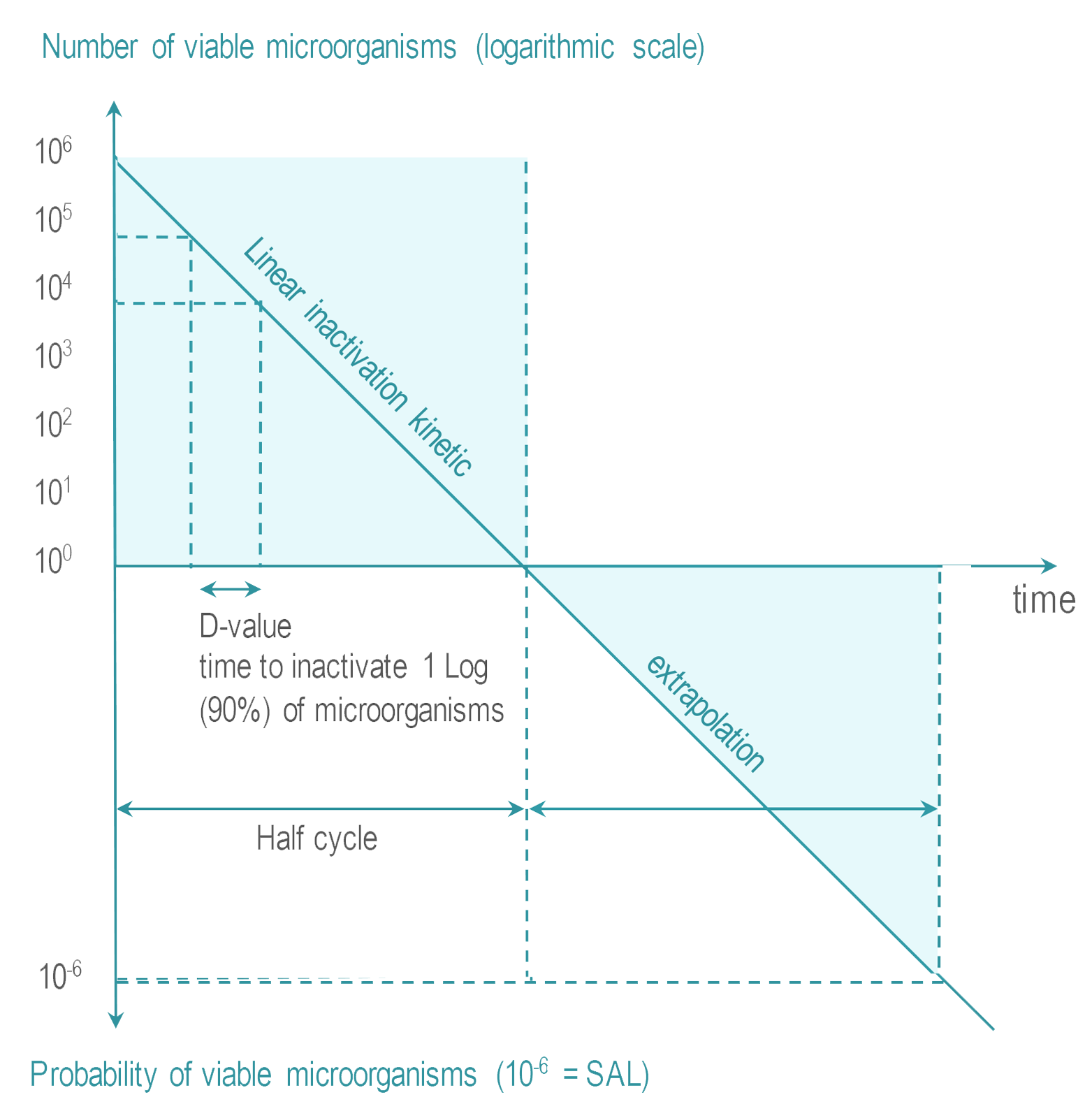 |
将 RDM 在包装内进行灭菌,并保持无菌状态直至使用前,这样的灭菌过程称为最终灭菌。
最终灭菌可以在高温或低温下进行。
蒸汽灭菌是最常见的灭菌方法。 也称为湿热灭菌或饱和蒸汽灭菌),
在干热灭菌中,RMD 暴露在干燥的热空气中。 低温灭菌 (LTS) 适用于不耐高温的 RMD。 目前的低温灭菌因子有:环氧乙烷 (EtO)、蒸汽甲醛 (LTSF)、汽化过氧化氢 (VH2O2) 和臭氧 (O3)。 在达到目标 SAL 所需的时间内,RMD 在受控的温度、湿度和/或压力条件下暴露于最低浓度 (Cc) 的灭菌因子。
|
![]() 辐射灭菌(电离 – 伽马、电子束或高能 X 射线,或非电离紫外线 (UV))通常不用于医疗机构中 RMD 的再处理,本指南中将不讨论。
辐射灭菌(电离 – 伽马、电子束或高能 X 射线,或非电离紫外线 (UV))通常不用于医疗机构中 RMD 的再处理,本指南中将不讨论。
非最终灭菌方法满足 SAL指标。然而,与最终灭菌不同,RDM 不受包装保护。即时使用蒸汽灭菌(以前称为快速灭菌)是非最终灭菌过程的一个例子。
实施所有灭菌过程都需要职业健康和安全的预防措施。
- 蒸汽灭菌器腔体中的高压,要求根据适用法规或专业指南对腔体完整性进行定期控制。
- 蒸汽和干热灭菌的高温有灼伤危险。 RMD需要有时间冷却,操作员应戴上手套。
- 所有低温化学品都具有不同程度的毒性(这就是它们对微生物有效的原因)。定期控制检查是否有泄漏。在接触 RMD 之前,残留物应减少至相应职业健康和安全法规规定的水平。
市场上有各种尺寸和配置的灭菌器。
- 中央灭菌部门使用大型灭菌器。 通常是双门(直通)类型。
- 台式、单门灭菌器用于门诊、牙科和农村诊所。
根据 Spaulding 分类原则和适用的当地法规或专业指南选择灭菌方法。
常见的选择原则或技术趋势可概括如下:
- 当RMD 进入人体无菌腔道时,最终灭菌优于非最终灭菌。
- 对于耐受湿热的RMD,推荐使用蒸汽灭菌。最有效的蒸汽周期是 132°C (270°F) 或 134°C 的周期。根据适用法规,要求或推荐的维持时间从 3 分钟到 18 分钟不等。
- 干热灭菌由于其固定蛋白质性质和与蒸汽灭菌相比的性能较差,而在越来越多的国家被禁止。
- 对于热敏 RMD,终末低温灭菌 (LTS) 方法的选择遵循 RMD 制造商的 IFU,可能会受到地区公约、指南或法规的影响。汽化过氧化氢 (VH2O2) 是最广泛接受的 LTS 方法。低温蒸汽甲醛 (LTSF) 因其固定蛋白质特性而未在朊毒体监管严格的国家/地区使用。由于固定特性、解吸时间长以及职业健康和安全限制,EtO 的使用取决于国家/地区。
- 部分国家不使用非终末IUSS。但它在其他国家中仍然允许,但通常推荐使用终末蒸汽灭菌。
- 非终末液体灭菌过程的接受程度取决于地区。在液体灭菌过程中,RMD 被浸入产生 10-6 SAL 的溶液中。然后接着冲洗 RMD。 RMD 不受包装保护。因此,液体灭菌剂过程是非终末的。
用户可以灵活选择灭菌方法,这取决于当地法规或指南。例如,在某些国家/地区会使用蒸汽,除非 RMD 制造商的 IFU 不允许。在一些国家/地区,LTS 用于耐受蒸汽的器械(例如,光学器件)避免反复暴露于蒸汽而造成性能下降。
当地法规可能要求灭菌过程同时符合国际标准。
![]() 蒸汽1、2、3、4、5 干热6、EtO7、8 和LTSF9、10 有专用标准。 ISO 14937 用于 VH2O2(目前正在制定专用标准)12,13。 没有直接适用于液体灭菌剂过程和 IUSS 的国际标准。
蒸汽1、2、3、4、5 干热6、EtO7、8 和LTSF9、10 有专用标准。 ISO 14937 用于 VH2O2(目前正在制定专用标准)12,13。 没有直接适用于液体灭菌剂过程和 IUSS 的国际标准。
灭菌周期可分为 3 个阶段。
- 前处理阶段是准备灭菌室和包装的 RMD的条件,以实现高效灭菌。例如,使腔体温度达到预定水平,并且通过真空去除阻碍灭菌因子和RMD表面接触的空气。程序曲线各不相同(例如,一个真空或几个真空)。对于某些灭菌技术(例如 EtO),灭菌周期的前处理阶段之前可能会进行预处理(例如,使 RMD 处于预定的温度和湿度范围内)。
- 在预定时间和受控条件下接触灭菌因子可达到 SAL。暴露阶段包括灭菌因子在腔体中分布、通过包装以及接触 RMD 的所有表面(包括中空几何空间)所需的时间。
- 去除灭菌残留以便安全地打开腔门。从吸收性材料中解吸有毒化学物质可能需要较长的通风阶段(例如,在 EtO 的情况下)。无论采用何种灭菌技术,都应明确并严格执行职业健康和安全措施。经过滤的空气进入腔体以消除冷凝(来自蒸汽)或清除腔体和负载表面的有毒残留物。可以通过压力、T°C 变化或其他方式来帮助去除残留物。经过高温灭菌,RMD 可以允许进行冷却。如果需要,周期进行期间或完成之后应有解吸阶段以去除负载中有毒残留物。
书面标准操作程序 (SOP’s) 描述了在灭菌周期之前、期间和之后要执行的操作和控制。 对于新购买的RMD,如果现有的SOP不适用,则定义新的SOP。
- 系统检查耗材(灭菌剂和其他)的有效期。 根据制造商的 IFU 和当地法规,超过保质期的产品应丢弃。
- 负载组成是预先定义的。
每件物品都带有一个化学指示物 (CI)。 国际标准 ISO 11140-114 将化学指示物分为 6 种类型(没有等级意义),如表 1 所示。大多数当地指南要求使用 1 类 CI。 一些指南建议增加 3、4、5 或 6 类指示物。 表格1。
| 类型 | 名称 | 描述 |
| 1 | 过程指示物 | 预期用于单个单元(灭菌包或灭菌盒),区分经过灭菌处理过程和未经过灭菌处理过程的单元。一类指示物通常整合在包装材料中或作为包装的附件。 |
| 2 | 用于特定测试的指示物 | 预期用于特定的测试。如用于测试预真空灭菌程序中空气去除效果的B-D 测试。不同的B-D测试指示物在国际标准ISO11140 -3 到 ISO 11140-6 各部分有详细描述。 |
| 3 | 单变量指示物 | 对灭菌变量中的其中一个起反应,如时间和温度。并预期用于表达该变量达到了标定值的要求, 如134度。 |
| 4 | 多变量指示物 | 对灭菌变量中的其中两个或多个起反应,如时间和温度。并预期用于表达该变量达到了标定值的要求, 如134度, 3分钟。 |
| 5 | 整合指示物 | 对所有灭菌变量起反应,如时间,温度和水蒸汽。并预期等同或超过ISO11138系列标准(见后面章节)所给出的对生物指示物的性能要求。 |
| 6 | 模拟指示物 | 对所有关键灭菌变量起反应,如时间,温度和水蒸汽。并预期符合特定灭菌周期的要求。 |
- 生物指示物 (BI) 的使用取决于一个国家的法规或指南以及灭菌技术。 BI符合国际标准19、20、21、22、23、24 生物指示物通常是自含式的细菌指示物(SCBI)
在 自含式生物指示物SCBI 中,用于培养和恢复测试微生物所需的生物指示物和培养基被封闭在无菌屏障系统中。暴露后,介质在无菌条件下与 BI 接触。 SCBI 提供的结果是二选一:生长或没有生长。 读取时间从 48 小时到几小时不等,新一代 BI 甚至更短。快速阅读型 BI 可检测到可靠地模拟测试微生物生长反应的酶 |
- 负载布置有利于灭菌因子均匀分布。 装载应考虑到职业健康和安全。
- 日常控制(如果需要)按国际标准推荐或当地规范定义的期限进行。
- 依据负载选择相应周期。 周期控制是自动化的,但工作人员必须在场并接受培训,以便对警报和危险情况做出反应。 国际标准要求有检测控制系统偏差的方法和独立记录周期数据。
灭菌器制造商必须提供措施以避免控制系统的软件或硬件故障未被检测到,导致无效周期看起来“有效”。 在实践中,独立传感器会相互检查过程变量是否在规定的公差范围内。 例如,在蒸汽灭菌的情况下,控制过程温度的温度传感器由同一位置的另一个独立的温度传感器监测。 独立传感器技术不需要相同,只要可靠地检测到控制功能的偏差即可。 制造商的 IFU应会向用户提供指引,当检测到偏差的处理措施。 必须记录独立数据以进行追溯。 |
- 准确描述周期结束后的控制措施,记录结果,记录偏差,并按照 SOP 中的规定进行报告。 每个灭菌包都会目视检查。 如果未在灭菌前进行,则补充物品标签以实现可追溯性。 检查周期参数。检查 1 类化学指示物的颜色变化和其他 CI(如果使用),BI(见下文)则送去培养。
- 开门、卸载功能完好。 遵循职业健康和安全措施防止接触有毒残留物和灼伤。
- 描述和分配产品放行的职责。 安排后备人员以确保流程的连续性。
| 灭菌器制造商必须提供措施以避免控制系统的软件或硬件故障未被检测到,导致无效周期看起来“有效”。 在实践中,独立传感器会相互检查过程变量是否在规定的公差范围内。 例如,在蒸汽灭菌的情况下,控制过程温度的温度传感器由同一位置的另一个独立的温度传感器监测。 独立传感器技术不需要相同,只要可靠地检测到控制功能的偏差即可。 制造商的 IFU应会向用户提供指引,当检测到偏差的处理措施。 必须记录独立数据以进行追溯。 |
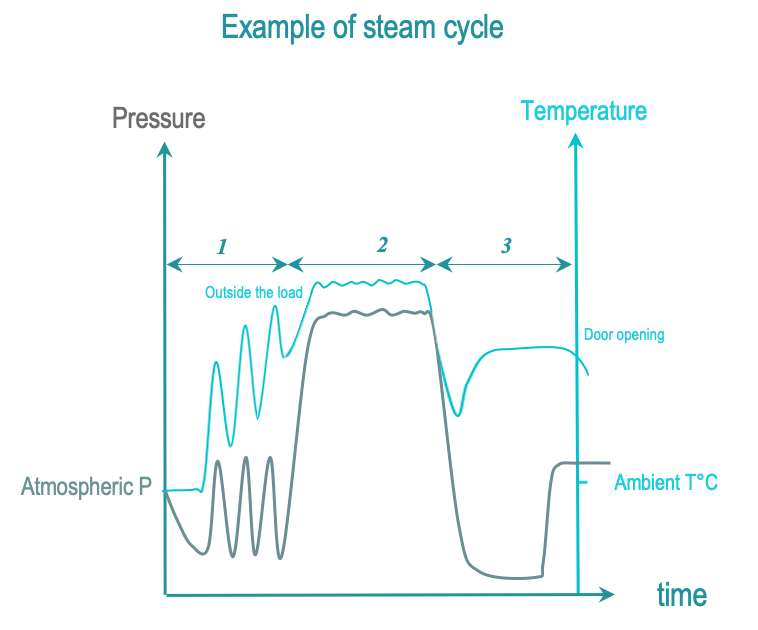
在蒸汽灭菌中,热饱和蒸汽在根据指南设定的时间内覆盖所有 RMD 表面。 饱和意味着蒸汽保持在液相和气相之间转换的稳定状态,且液相的百分比非常低。 Regnault 表给出了蒸汽饱和的压力和温度条件。
高于 100°C 的温度是通过将灭菌室中的压力增加到大气压以上来获得的。
根据国际标准的推荐或当地法规的规定定期(通常每天)进行渗透测试和泄漏测试。
蒸汽灭菌周期的 3 个阶段如下:
- 前处理:连续抽真空和注入蒸汽,从腔体和负载中抽出空气并替换为饱和蒸汽。负载温度逐渐升高。
- 暴露:饱和蒸汽注入完成并在给定时间内扩散至负载。 RMD 的所有表面都必须暴露在饱和蒸汽中。暴露温度和时间由当地法规或指南定义。常用温度为 134°C 或 132°C (270°F),具体取决于地区。维持时间从 3 到 18 分钟不等(由于朊毒体的监管要求)。在灭菌阶段,冷凝和温度下降通过饱和蒸汽注入进行补偿,直到灭菌时间结束。当 RMD 制造商的 IFU 指定时,温度可降低到 121°C 或 125°C,维持时间为 15 分钟或更长。
后处理:通过真空和加热去除冷凝物。打开门让负载冷却,以便操作员可以安全地处理负载。
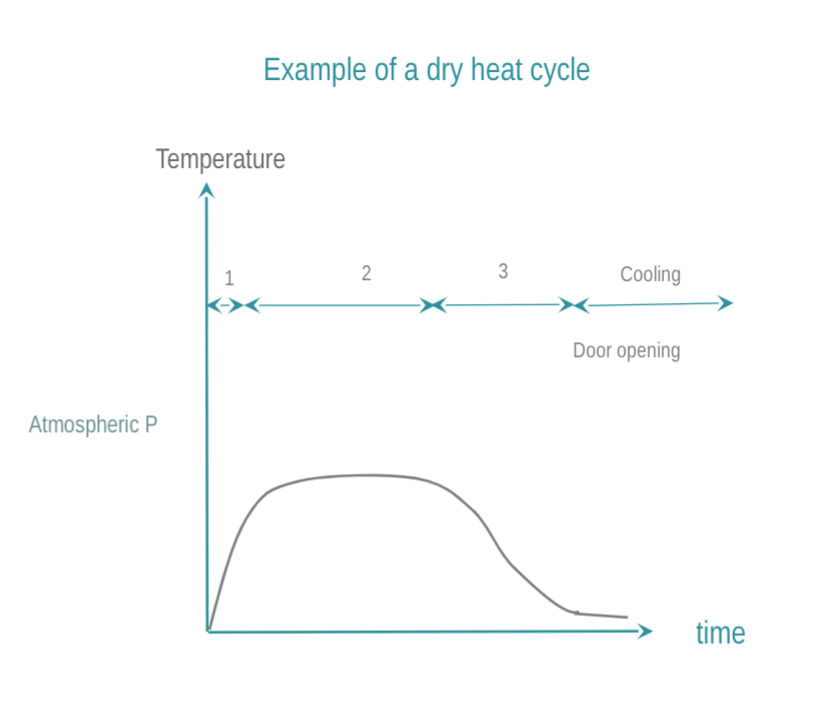
干热通过热传导实现灭菌。热量被 RMD 吸收并在 RMD 内逐层移动。要使 RMD 完全灭菌,需要达到所需的温度。与蒸汽灭菌相比,干热T°C和起效所需时间更高更长,渗透性能差,冷却时间更长。由于其固定蛋白质的特性,越来越多的国家禁止干热。如何进行干式灭菌:
- 腔体升温到目标温度。优先选用在整个灭菌室中有强制气流进行干热灭菌的方式。
- RMD 在目标温度下保持预定时间。常用温度和时间为 160 °C (320 °F) 2 小时,或 170 °C (340 °F) 1 小时,或者在高速热气流灭菌器的情况下为 190°C。
- 为了操作员的安全,腔体和负载保证有时间冷却。打开门后可以延长冷却时间。
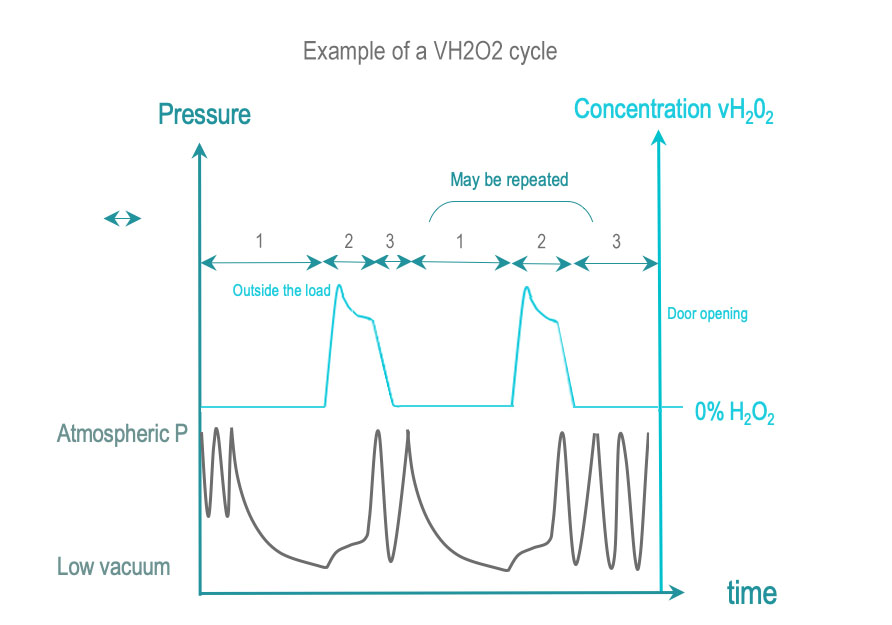
在 SAL 所需的处理时间内,汽化过氧化氢灭菌使 RMD 表面与一定浓度的汽化过氧化氢 (VH2O2) 接触。 VH2O2 是通过液态灭菌剂溶液(常见的浓度在 50% 以上)汽化获得的。 一些周期会在VH2O2进入腔体之前增加其浓度。
- 前处理:通过单次或重复真空除了去除空气外,也可结合其他方法一起去除物品的残留湿气。 控制负载处于预先确定的温度范围内。
- 暴露:汽化的 H2O2 被注入并扩散到腔体、通过包装到达负载及其复杂的几何空间内。 H2O2 在起反应时或被材料吸收时会分解。 一定剂量的 H2O2 被输送到腔体,并确保所有表面都暴露在 SAL 所需的时间和浓度条件下。直接监测 H2O2 浓度或通过压力间接监测。
- 后处理:通过真空或其他方式去除残留或吸附在负载上的水和H2O2残留物
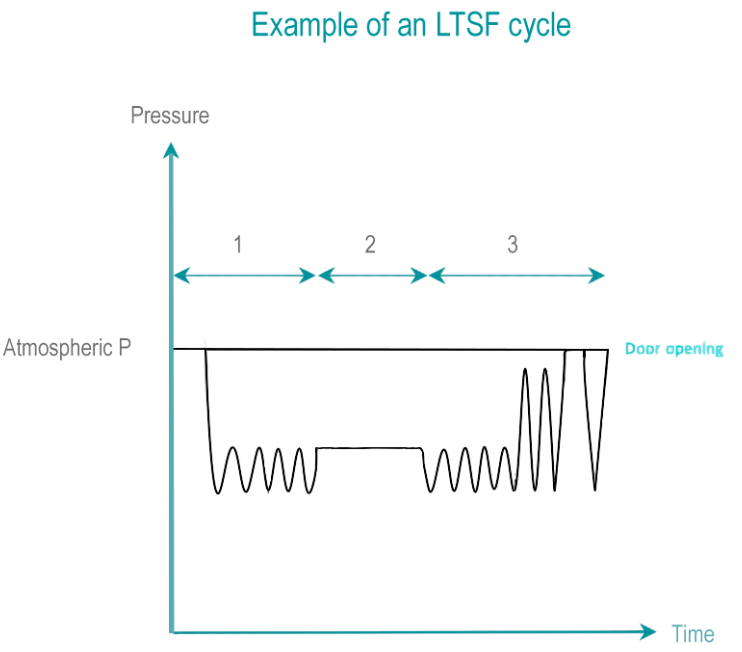
甲醛 (HCHO) 是一种无色气体,极易溶于水。 甲醛是通过将不同浓度甲醛(低于 40%)的溶液蒸发而获得的。 湿度的存在大大提高了甲醛的灭活能力。
- 前处理:从抽真空开始从腔体和负载中去除空气。 使用压力脉冲,残留空气会逐渐被蒸汽和甲醛取代。
- 暴露:在暴露时间内,温度、灭菌剂浓度、压力和湿度均保持在预定范围内。 LTSF 在 80、65、60、55 或 50°C(176、149、140、131 或 122°F)下运行,相对湿度为 75% 至 100%。
- 后处理:通过反复抽真空和蒸汽冲洗,然后是深度真空,将甲醛和蒸汽混合物从负载中去除。 解吸阶段可使甲醛残留从 RMD去除到对操作者和病人无害的水平。 液态甲醛残留被稀释到当地废物监管允许的水平。
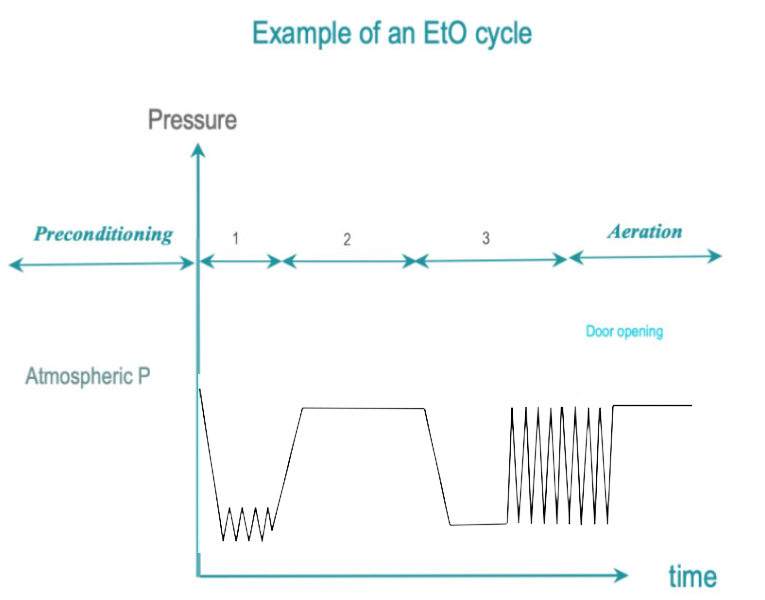
EtO 是一种无色有毒气体,可攻击微生物的细胞蛋白质和核酸。 EtO 过程温度范围为 25 – 55°C。较低的温度会导致效率较低的过程和较长的暴露时间。 EtO 对人类具有致癌性且易燃。需要特殊的房间条件、安全设备和单独的通风系统。
- 前处理:残余空气通过真空脉冲去除,并逐渐被 EtO 与蒸汽混合取代。负载的温度和湿度被调整到预定的水平。对于大型负载可能需要进行预处理,使其处于预定的湿度和温度范围内。
- 暴露: EtO 浓度、湿度和温度稳定在设计值内,直到预定的暴露时间结束。
- 后处理:通过真空脉冲去除 EtO 残留物。在解吸阶段,保证从负载中去除EtO的时间。在严格符合职业健康和安全的条件下,可以在灭菌腔体外继续解吸过程。总解吸时间在 12 到 48 小时之间。
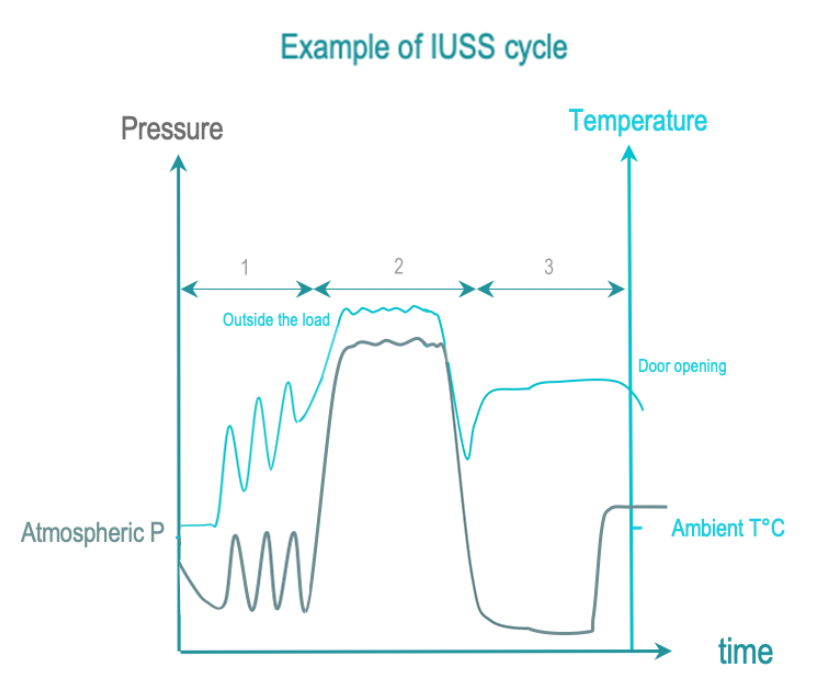
即时使用蒸汽灭菌 (IUSS) 是蒸汽灭菌的一种,用于使用点紧急的非最终灭菌。 RMD是裸露的。立即转移给使用者,并在受控环境中小心实施。与蒸汽灭菌相比,RMD 没有包装,干燥和冷却通常会缩短以限制周期时间。因此,RMD 可能在周期完成时仍然是湿的,从而增加了受环境污染的风险。
IUSS 的 3 个阶段如下:
前处理:真空和蒸汽注入,从腔体和负载中抽出空气,用饱和蒸汽代替。负载温度逐渐升高。
暴露:饱和蒸汽注入完成并保证在整个负载中扩散的时间。
后处理:通过真空和加热去除冷凝物。在打开门后允许负载冷却使操作员安全处理。
一些国家禁止 IUSS,其他国家可能会允许。专业指南通常建议对在使用点处理的必要性进行评估。 RMD备用数量可能需要调整才能支持集中处理。
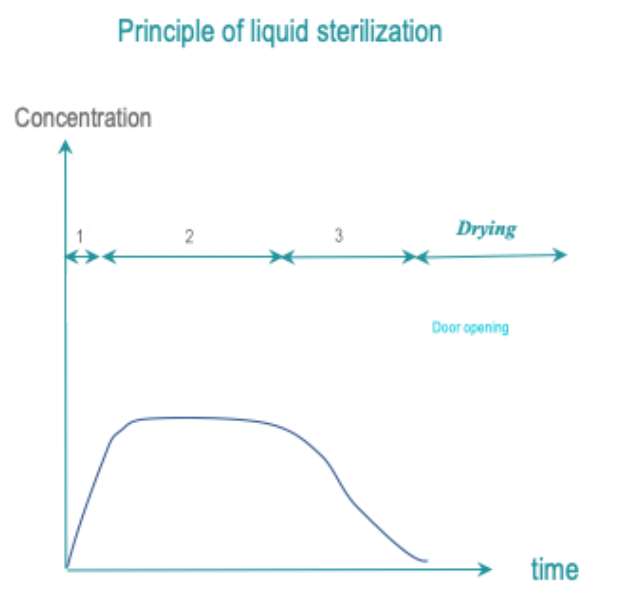
将RMD所有表面暴露于液体灭菌剂中以控制时间、温度和浓度,从而达到目标 SAL。 冲洗必须保持 SAL。
最常见的灭菌剂是过氧乙酸。 清洁步骤通常与灭菌剂应用分开。
接受液体灭菌剂的概念取决于地区。 在某些国家/地区,它可能被允许作为某些易碎或热敏感 RMD 的高温或低温灭菌之外的替代方法。 在其他国家,这种做法可能被认为是背离了 Spaulding 分类原则。
- 前处理阶段:可能不需要这个前处理阶段。 清洁步骤可能包含在周期中或单独进行。
- 暴露:RMD所有表面都在预定的温度和条件下暴露于灭菌剂中并持续预定的时间。
- 后处理:通过冲洗去除灭菌剂残留物。 冲洗应保持 SAL。 如果周期中包括干燥,干燥也保持 SAL。
书面的清洁和消毒标准操作程序 ( SOP’s) 是根据质量管理原则制定的。
用户监督或执行和控制过程验证:
- 灭菌器的安装符合制造商的专业推荐
- 可以获得IFU、测试和校准证书
- 标准操作程序 (SOP) 是最新的。 对于新购买的RMD,如果现有的SOP不适用,则定义新的SOP。
- 常规控制到位
- 职业健康和安全措施到位
- 执行最新内容的培训(包括职业健康和安全措施培训),并且有培训证书。
- 所有灭菌设备都有维护计划
- 追溯是可操作的

WFHSS 清洁和消毒的关键建议
- 最终灭菌,符合国际标准,优于非最终灭菌。
根据最终灭菌方法,134°C 或 132°C 的蒸汽是首选的灭菌温度,在 RMD 制造商的 IFU 指定时即可使用。维持时间因国家/地区法规或指南而异。
干热灭菌应用蒸汽灭菌替代。
对于与 132°C 或 134°C蒸汽不兼容的 RMD,医疗器械制造商的 IFU应指明在 121°C 至 125°C 蒸汽灭菌或低温灭菌中可以使用的灭菌方法。 LTS 方法的选择可根据法规、指南、实践或职业健康和安全来考虑。
液体灭菌的相关声明仍有待评估。
获得无菌和安全的器械需要根据质量管理原则以及 RMD 和灭菌器制造商的 IFU来定义过程验证。
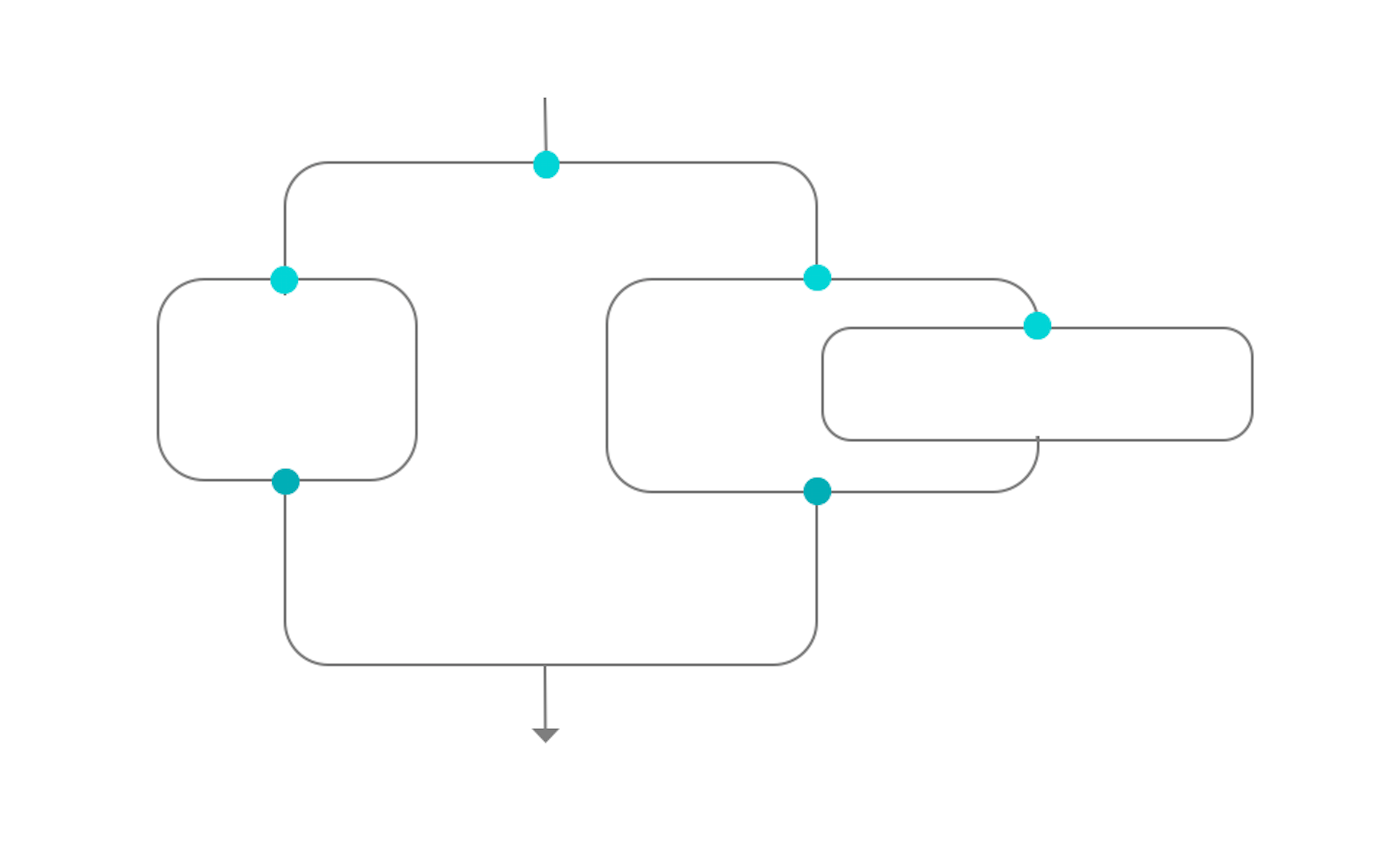
IUSS to be replaced by steam sterilization
Go to IUSS sterilization →
1 of 16 液体灭菌剂To be evaluated by WFHSS
Go to Liquid sterilization →
2 of 16 蒸汽Steam 134°C or 132°C preferred when allowed by RMD IFU
Go to Steam sterilization →
3 of 16 低温蒸汽甲醛Cycle according to RMD IFU
Go to Low temperature steam formaldehyde →
4 of 16 汽化过氧化氢Cycle according to RMD IFU
Go to Vaporized H2O2 →
5 of 16 环氧乙烷Cycle according to RMD IFU
Go to Ethylene Oxide →
6 of 16 清洁、干燥、打包的 RMDNon packaged for non terminal sterilization
Go to choice of sterilization process →
7 of 16 无菌医疗器械Non packaged RMD for immediate use when non terminal sterilization
Packaged RMD for storage when terminal sterilization
Go to choice of sterilization process →
Terminal sterilization preferred to
non terminal
Go to choice of sterilization process →
9 of 16 非最终灭菌The RMD is not protected by a packaging and must be immediately used after sterilization
Go to choice of sterilization process →
10 of 16 +Terminal sterilization preferred
Go to recommendation of WFHSS for sterilization →
11 of 16 +Steam sterilization at 132°C or 134°C preferred when allowed by RMD IFU
Go to recommendation of WFHSS for sterilization →
12 of 16 +Visual control and routine controls
Go to Sterilization and quality management →
13 of 16 +According to RMD IFU
Go to choice of sterilization process →
14 of 16 +Steam sterilization at 132°C or 134°C preferred when allowed by RMD IFU
Go to recommendation of WFHSS for sterilization →
15 of 16 +Visual control and routine controls
Go to Sterilization and quality management →
16 of 16- ISO 17665-1:医疗保健产品灭菌 – 湿热 – 医疗器械灭菌过程的开发、确认和常规控制要求 – 2006
- ISO 17665-2:卫生保健产品的灭菌 – 湿热 – 第 2 部分:ISO 17665-1 – 2009 应用指南
- ISO 17665-3:医疗保健产品灭菌 – 湿热 – 第 3 部分:将医疗器械指定为蒸汽灭菌产品族和过程类别的指南 – 2013
- EN 285:灭菌——蒸汽灭菌器——大型灭菌器——2016
- EN 13060:灭菌——蒸汽灭菌器——小型蒸汽灭菌器——2018
- ISO 20857:医疗保健产品灭菌 – 干热 – 医疗器械灭菌过程的开发、确认和常规控制要求 – 2014
- ISO 11135:医疗保健产品灭菌 – 环氧乙烷 – 医疗器械灭菌过程的开发、确认和常规控制要求 – 2014
- EN 1422:医用灭菌器——环氧乙烷灭菌器——要求和测试方法——2014
- ISO 25424:保健品灭菌——低温蒸汽和甲醛——医疗器械灭菌过程的开发、确认和常规控制要求——2018
- ISO 14937:医疗保健产品的灭菌——灭菌因子特性以及医疗器械灭菌过程的开发、确认和常规控制——2009
- EN 14180:低温蒸汽甲醛灭菌器——要求和测试
- ISO 22441:医疗保健产品的灭菌——低温汽化过氧化氢——医疗器械灭菌过程的开发、确认和常规控制的要求——(正在进行中)
- EN 14180:医用灭菌器——低温过氧化氢灭菌器——要求和测试(进行中)
- ISO 11140-1:医疗保健产品灭菌 – 化学指示物 – 第 1 部分:通则 – 2014
- ISO 11140-3:医疗保健产品的灭菌 – 化学指示物 – 第 3 部分:用于 BD类蒸汽渗透测试的 2 类指示物系统 – 2007
- ISO 11140-4 医疗保健产品灭菌 – 化学指示物 – 第 4 部分:用于替代BD类蒸汽渗透测试的 2 类指示物 – 2007
- ISO 11140-5 保健产品灭菌 – 化学指示物 – 第 5 部分:用于BD类空气排除测试的 2 类指示物 – 2007
- ISO 11140-6 医疗保健产品灭菌 – 化学指示物 – 第 6 部分:用于蒸汽灭菌器性能测试的 2 类指示物和过程挑战装置(正在进行中)
- ISO 11138-1:保健产品的灭菌 – 生物指示物 – 第 1 部分:通则 – 2017
- ISO 11138-2:保健产品的灭菌——生物指示物——第 2 部分:环氧乙烷灭菌用生物指示物——2017
- ISO 11138-3:保健产品的灭菌——生物指示物——第 3 部分:湿热灭菌用生物指示物——2006
- ISO 11138-4:保健产品的灭菌——生物指示物——第 4 部分:干热灭菌用生物指示物——2017
- ISO 11138-5:保健品灭菌——生物指示物——第5部分:低温蒸汽和甲醛灭菌用生物指示物——2017
- ISO/AWI 11138-6:保健产品的灭菌——生物指示物——第6部分:汽化过氧化氢灭菌用生物指示物(进行中)













