Sterilization is intended to render Reusable Medical Devices free from viable mircroorganisms
![]() Prion are not microorganisms and are more resistant than conventional microorganisms.
Prion are not microorganisms and are more resistant than conventional microorganisms.
Sterilization of RMD is based on 3 key concepts
- Sterility Assurance Level (SAL) : It is not possible to systematically control the total absence of viable microrganism. The objective of sterilization is to limit the probability of microorganism survival to a very low level. For RMD’s the probability or Sterility Assurance Level (SAL) is expressed as: not more than 1 viable microorganisms in an amount of one million sterilized items (or 10-6 SAL ).
- Overkill : The quantity, nature and location of microorganisms which may be present on an RMD after cleaning is not known. RMD sterilization processes must demonstrate their ability to inactivate a highly concentrated inoculum of > 1 million of test microorganisms. Test microorganisms are selected for their high resistance to the sterilization process. This conservative margin above the highest level of real life contamination is called overkill method.
- Compatibility: RMD’s remains fully functional and safe for use after sterilization. Compatibility is checked by RMD Manufacturer, which determines the maximal number of sterilization cycles to which an RMD can be exposed before being discarded or repaired.
Various methods are proposed by international standards to demonstrate the capability of a given sterilization process to reach the SAL using overkill method. One of them is the half cycle overkill method, described as follows:
Tests are performed with microorganisms known for their high resistance to the sterilization process (usually bacterial spores).
Once the process has been characterized, it must be verified that an RMD can be efficiently sterilized. The 106 inoculum is placed at the position determined as the most difficult to sterilize on or within an RMD. RMD's are packaged and inserted in a representative challenging load. | 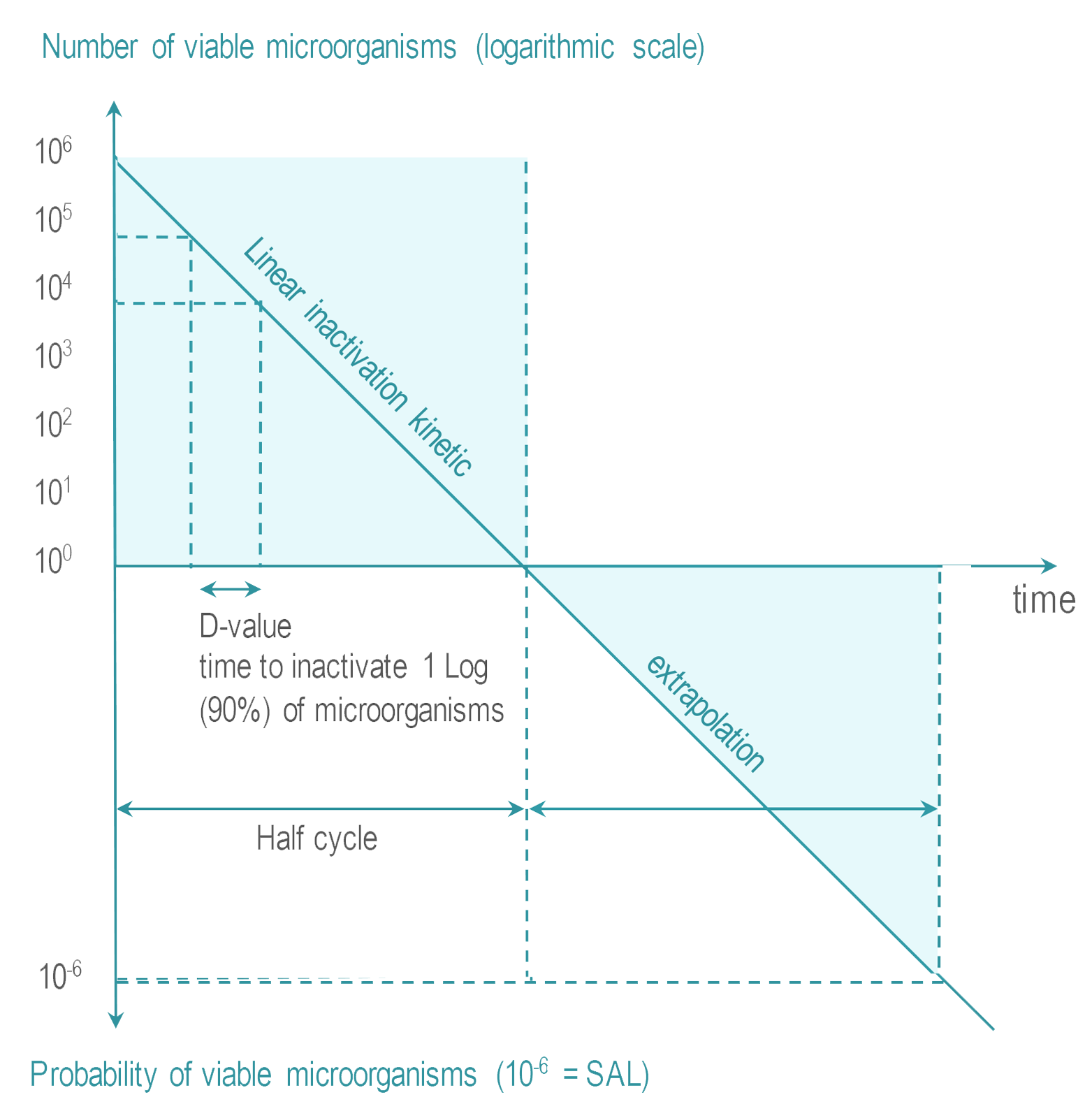 |
The sterilization process whereby an RMD is sterilized within a packaging which preserves its sterility until use is called terminal sterilization.
Terminal sterilization can be obtained at either high temperature or at low temperatures.
Steam sterilization is the most common sterilization method. It is also referred to as moist heat sterilization or saturated steam sterilization),
In dry heat sterilization, RMD are exposed to dry, hot air. Low temperature sterilization (LTS) is adapted tor RMD's, which do not withstand high temperatures. Current low temperature sterilizing agents are: Ethylene Oxide (EtO), steam formaldehyde (LTSF), vaporized hydrogen peroxide (VH2O2). RMD are exposed in controlled temperature, humidity and/or pressure conditions to a minimum concentration (Cc) of sterilizing agent during the time required for achieving the desired SAL.
|
![]() Radiation sterilization (ionizing – gamma, e-beam or high energy X-Ray, or non-ionizing ultra-violet (UV) ) is not commonly used for reprocessing of RMD’s in healthcare facilities and will not be discussed in present guidelines.
Radiation sterilization (ionizing – gamma, e-beam or high energy X-Ray, or non-ionizing ultra-violet (UV) ) is not commonly used for reprocessing of RMD’s in healthcare facilities and will not be discussed in present guidelines.
Non-terminal sterilization methods meet the SAL criteria. However, unlike terminal sterilization, RMD’s are not protected by a packaging. Immediate use steam sterilization (previously called flash sterilization) is an example of non-terminal sterilization process .
All sterilization processes require occupational health and safety precautions.
- High pressure in steam sterilizer chambers requires periodic control of chamber integrity, according to applicable regulation or recommendations.
- High temperature of steam and dry heat sterilization expose operators to risk of burns. RMD’s are given time to cool down and operators wear gloves.
- All low temperature chemicals are toxic at various levels (this is why they are efficient on microorganisms). Periodic controls check the absence of leaks. Before accessing an RMD, residues are eliminated to levels defined by applicable occupational health and safety regulations.
Various and size and configurations of sterilizers are offered on the market.
- Large sterilizers are used in central sterilization facilities. There are often double door (pass-through) systems.
- Table top, single door sterilizers are used in outpatient, dental and rural clinics.
A sterilization method choice is made according to Spaulding classification principles and applicable local regulations or recommendations.
Common preferences or trends may be summarized as follows :
- Terminal sterilization is preferred to non terminal sterilization for RMD’s entering sterile cavities.
- Steam sterilization is recommended for moist and heat compatible RMD’s. The most efficient steam cycles are those at 132°C (270°F) or 134°C. Required or recommended holding time varies from 3 minutes, up to 18 minutes according to applicable regulation.
- Dry heat sterilization is banned in a growing number of countries due to its fixative properties and poor performances compared to steam sterilization.
- For heat sensitive RMD’s, choice of the terminal low temperature sterilization (LTS) method follows RMD manufacturers’ IFU’s and may be influenced by regional conventions, guidelines, or regulations. Vaporized hydrogen peroxide (VH2O2) is the most widely accepted LTS method. Low Temperature Steam Formaldehyde (LTSF) is not used in countries with strong prion regulation, due to its fixative properties. Use of EtO is country dependent due to fixative properties, long aeration time and occupational health and safety constraints.
- Non terminal IUSS is not used in some countries. It remains tolerated in others, but generally with recommendations to use terminal steam sterilization.
Level of flexibility is left to user for choice of the sterilization method, which depends on local regulations or guidelines. For instance, in some countries steam, is used except when not allowed by an RMD manufacturer’s IFU. In other countries, LTS is used or tolerated for steam-compatible devices known to deteriorate from repeated exposure to steam (e.g., optics).
Compliance of a sterilization process to international standards may be required by local applicable regulations or guidelines.
Sterilization cycles can be divided into 3 phases.
- Conditioning prepares the sterilization chamber and a packaged RMD for efficient sterilization. For example, the chamber temperature is brought to a predetermined level, and air acting as a buffer between the sterilizing agent and RMD surfaces is removed by vacuum. Conditionning profiles vary (e.g., one vacuum or several vacuum phases). With some sterilization technologies (e.g., EtO), the conditioning phase of the sterilization cycle may be preceded by a preconditioning (e.g., to bring an RMD within a predefined temperature and humidity range).
- Exposure to the sterilizing agent for a predefined time and controlled conditions achieves the SAL. Exposure includes the time needed to distribute the sterilizing agent in the chamber, through the packaging and on all surfaces of an RMD including hollow geometries.
- Removal of sterilization residues allows the chamber door to be opened safely. A long aeration phase might be needed for desorption of toxic chemical from absorbent material (e.g., in the case of EtO). Whatever the sterilization technology, appropriate occupational health and safety measures are defined and strictly applied. Filtered air is admitted in the chamber to eliminate condensation (from steam) or to purge toxic residues from the chamber and load surfaces. Removal of residues might be assisted by pressure, or T°C variation, or other means. With high temperature sterilization, RMD’s are allowed to cool down. If needed, aeration during or after the cycle permits desorption of toxic residues from the load.
Written standard operating procedures (SOP’s) describe operation and controls to be performed before, during and after the sterilization cycle. For a newly purchased RMD, a new SOP is defined if an existing one cannot be used.
- Expiration date of consumables (sterilant and others) is systematically checked. Product above expiration date is discarded according to a manufacturer’s IFU and local regulations.
- Load composition is predefined.
- Each item carries a chemical indicator (CI). International standard classify chemical indicators in 6 types (with no hierarchical significance). Most local guidelines request a type 1 CI.
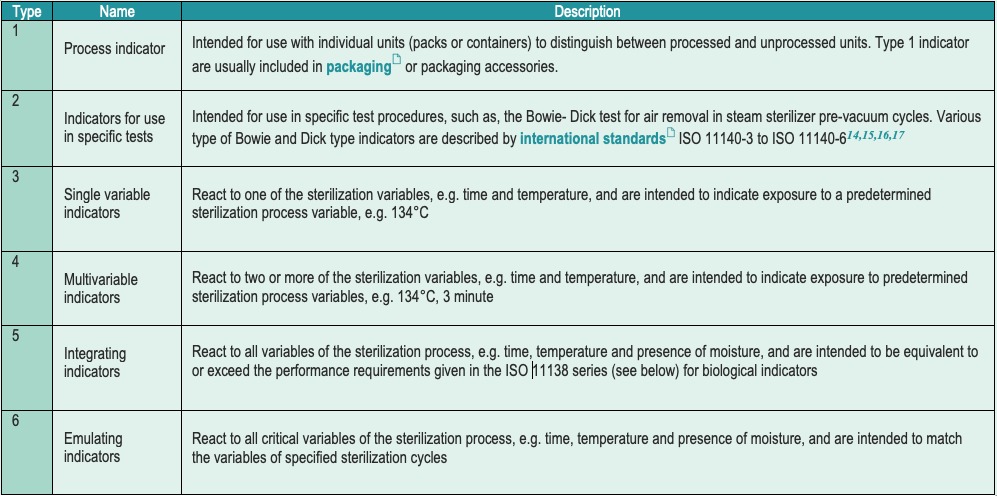
- Use of biological indicators (BI) depends on a country’s regulations, or guidelines, and sterilization technology. BI’s comply with international standards.
Biological indicators are usually self contained bacteriological indicators (SCBI)
| In an SCBI the biological indicator and medium required for incubation and recovery of the test microorganisms are enclosed in a sterile barrier system. After exposure, the medium is aseptically brought in contact with the BI. The answer provided by the SCBI is a binary growth no growth. Readout time ranges from 48 hours down to a few hours and even less with the new generation of BI. Fast readout BI detect enzymes which reliably simulates the behaviors of the test microorganisms. The SCBI often includes a process challenging device function. A process challenge device is defined by international standard ISO 11139 (2018) as "an item providing a defined resistance to a cleaning, disinfection, or sterilization process and used to assess performance of the process". In the case of an SCBI a tortuous path challenges the penetration of the sterilizing agent into the capsule containing the BI. To be meaningful the PCD challenge must be benchmarked against reusable medical devices intended for sterilization. BI's are placed in a challenging position in the load. |
- Load arrangement allow a uniform distribution of the sterilizing agent. Loading takes into account occupational health and safety considerations
- Routine controls (if needed) are performed at periodicity recommended by international standard or defined by local rules
- Cycles are adapted to the load. Control of cycle is automated but staff must be present and trained to react to alarm and hazardous situations. International standards require means to detect deviation of the control system and independent recording of cycle data.
| Means must be provided by a sterilizer manufacturer to avoid a software or hardware failure of the control system, which remains undetected, and that a non-valid cycle appears valid. In practice, independent sensors double-check that process variables are within specified tolerances. For instance, in the case of steam sterilization, the temperature sensor that regulates process temperature is doubled by an independent temperature sensor. The independent sensor technology does not need to be the same as long as deviation of the control function is reliably detected. A manufacturer's IFU provides instructions to a user in case of deviances are detected. Independent data must be recorded for traceability. |
- Post cycle controls are accurately described, results are recorded, and deviations are recorded, and reported as defined in an SOP. Each item is visually controlled. If not done before sterilization, labelling is implemented for traceability . Cycle parameters are controlled. Color changes of Type 1 Chemical indicators are controlled. Other CI (if used) are controlled and BI (see below) are sent for incubation
- Door opening and unloading functions are secured. Exposure to toxic residues and burns are prevented by following appropriate occupational health and safety measures.
- Load release responsibilities are described and assigned. Back-ups are organized to ensure process continuity.
| Load release (i.e. formal authorization to distribute the RDM for storage and use) is performed by an accredited person. The person in charge of load release is ideally different from the one in charge of unloading controls. Minimal requirements for load release are: Cycle parameters are within tolerances and no deviation of the control system are detected by the independent sensors Color changes of Type 1 CI are confirmed. Visual controls (damage to package, residual humidity,..etc..) are confirmed. Additional CI (other than type 1) may be required by the standard operating procedure (SOP). CI's placed in packaging are controlled at the point of use by operation room personnel. Results are not a prerequisite for load release. However, in case of a positive CI, a risk analysis is performed to determine whether some or all items must be reprocessed or recalled. When BI's are used, they are incubated and analyzed according to a BI manufacturer IFU. Healthcare facilities SOP's define measures in case of positive BI. A risk analysis determines if it is a false-positive (which is known to happen) or a true indication of an issue. Depending on the documented outcome of the risk analysis, the decision can be: a release of the load (because other control gave confidence that the cycle is conformed), a partial release or reprocessing of the entire load. Requirements for load release vary between countries and sterilization technologies. Parametric release (i.e., load release without a BI) is more common in Europe and usually linked to a more stringent observance of cycle parameters and process control. For saturated steam sterilization, it is universally accepted that, given the high margin safety, BI's are not systematically needed. Some countries like the US may recommend BI's for implants or advise daily or weekly BI controls. In most European countries, BI's are not used routinely for steam. For other sterilization technologies, activities depends on national best practices, or rules and interpretation of international standards. In any case, BI and CI are a complement to minimal requirements for load release as stated above but cannot be used to avoid control of cycles parameters, visual inspections and CI controls. |
In steam sterilization, hot saturated steam coats all RMD surfaces for a specific time set according to guidelines. Saturated means that steam is maintained at a steady state between a liquid and vapor phase with a very low percentage of the liquid phase. The Regnault table gives the pressure and temperature conditions for steam saturation.
Temperatures above 100°C, are obtained by increasing pressure in the sterilization chamber above atmospheric pressure.
A penetration test and a leak test are performed periodically as recommended by international standards, or imposed by local regulations (usually daily)
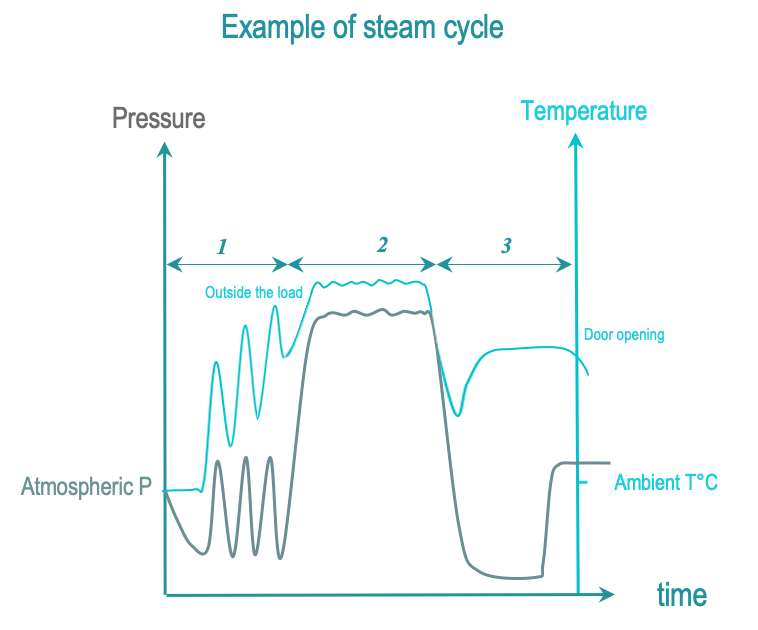
The 3 phases of steam sterilization cycles are as follows :
- Conditionning: Successive vacuum and steam injection withdraw air from the chamber and load and replace it with saturated steam. Load temperature is progressively increased.
- Exposure: Saturated steam injection is completed and given time to diffuse through the load. All surfaces of RMD must be exposed to the saturated steam. Exposure temperature and time are defined by local regulation or guidelines. Temperatures commonly used are 134°C or 132°C (270°F). Holding times vary from 3 to 18 minutes (in countries with prion regulation). Through the exposure phase, temperature decreases are compensated by saturated pressure injections until the end of a plateau period. When specified by an RMD manufacturer’s IFU, temperature is lowered at 121°C or 125°C with exposure times of 15 minutes or more. Condensation of saturated on sufaces and germs releases latent heat that significantly contributes to the efficacy of steam sterilization.
- Removal : Condensations is withdrawn by vacuum and heating. Open the door to allow the load to cool down so that the operator can handle the load safely.
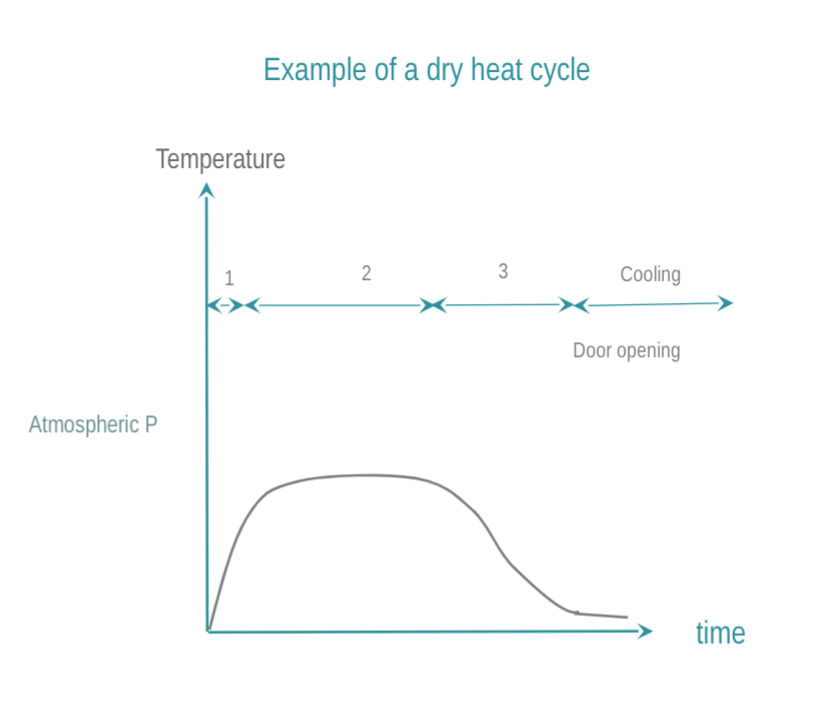
Dry heat achieves sterilization by means of conduction. The heat is absorbed by the RMD and moves within an RMD layer-by-layer. For the RMD to be fully sterilized, it needs to reach the required temperature. Compared to steam sterilization, dry heat T°C and time required for efficacy are higher and longer, the penetration performances are poor, and cool down-times are longer. Dry heat is banned in a growing number of countries due to its fixative properties. How to conduct dry sterilization:
- The chamber is brought to the targeted temperature. Dry heat is preferably forced throughout the sterilization chamber.
- An RMD is maintained at the target temperature for a predetermined time. Common temperature and time are 160 °C (320 °F) for 2 hours, or 170 °C (340 °F) for 1 hour, or at 190°C in the case of High Velocity Hot Air sterilizers.
- The chamber and load are given time to cool down for operator safety. Cooling may be prolonged after opening the door.
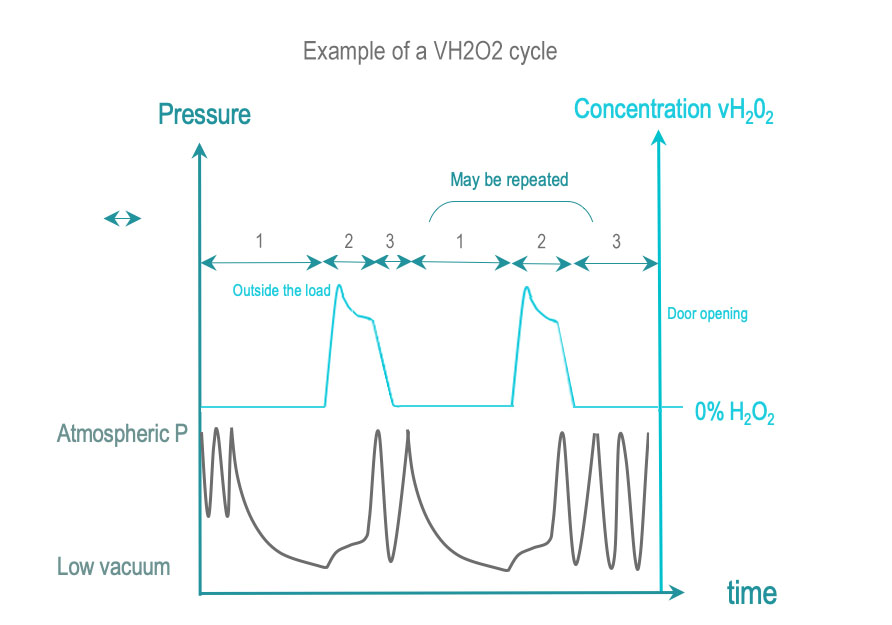
Vaporized hydrogen peroxide sterilization brings RMD surfaces in contact with vaporized H2O2 (VH2O2) at a certain concentration and during the process time required for SAL. VH2O2 is obtained by vaporization of a liquid sterilant solution (usually the concentration is above 50%). Some cycles increase the VH2O2 concentration before distribution in the chamber.
- Conditioning: Air is withdrawn by single or repeated low vacuums that contribute, to the removal of residual humidity traces. The load is brought within a predetermined temperature range.
- Exposure: Vaporized H2O2 is injected and diffuses in the chamber, through the load and the packaging, and in complex geometries. H2O2 condensates on surfaces and germs, thereby creating a very active layer of H2O2 that rapidly equilibrates with lower H2O2 concentration in gas phase. H2O2 concentration in gas phase decreases as it interacts or is absorbed by material. The dose of H2O2 delivered to the chamber and exposure time ensure that all surfaces are exposed to conditions required for SAL. H2O2 concentration is monitored directly or indirectly by pressure.
- Removal: Water and H2O2 residues left or adsorbed by the load are eliminated by vacuum or additional means
Conditioning, exposure and removal schemes may be repeated once or several times. Conditioning of repetition phases may be simplified compared to the initial conditioning. Usually the total number of repeat phase (including the first one) is even. The cycle ends with a final removal usually reinforced compared to previous removals.
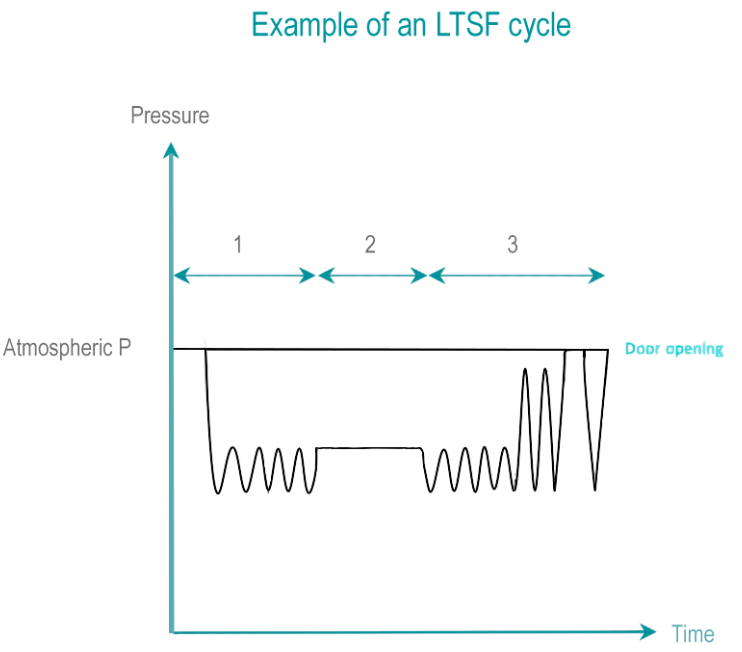
Formaldehyde (HCHO) is a colorless gas, classified as potentially carcinogenic. Formaldehyde is obtained by vaporization of a solution at various concentrations of formaldehyde (below 35%). Formaldehyde is highly soluble in water. Inactivation power of formaldehyde is greatly improved in presence of humidity.
- Conditioning: An initial vacuum removes air from the chamber and load. Residual air is progressively replaced by pulses of steam and formaldehyde. A liquid layer of condensate forms on surfaces of devices.
- Exposure time: During the exposure time, the temperature, sterilant concentration, pressure and humidity are all maintained within predetermined ranges. LTSF operates at 80, 65, 60, 55 or 50° C (176, 149, 140, 131 or 122° F) with a relative humidity of 75 to 100 %. Interaction with germs takes place in condensate, in liquid form.
- Removal: The formaldehyde and steam mixture is removed from the load by a repetition of vacuum and steam flushes. An Aeration phase allows desorption of formaldehyde from the load to levels that will not be harmful for operators and patients. Liquid formaldehyde residuals are diluted to level allowed by local waste management rules.
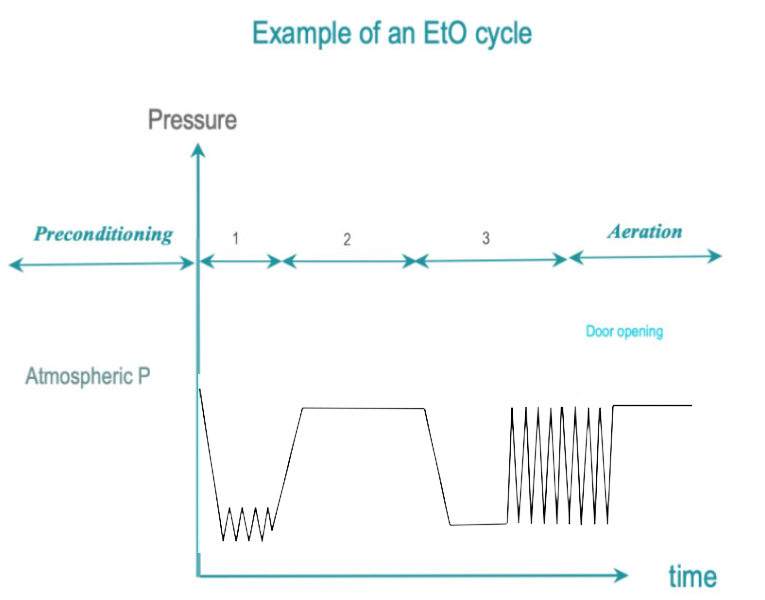
EtO is a colorless, highly penetrative, poisonous gas that attacks the cellular proteins and nucleic acids of microorganisms. EtO process temperatures range from 25 – 55°C. A lower temperature results in a less efficient process and a longer exposure time. EtO is carcinogenic to humans and flammable. Special room conditions, safety equipment and separate ventilation systems are required.
- Conditioning: Residual air is removed by vacuum pulses and progressively replaced by EtO mixed with steam. Temperature and humidity of the load are adjusted to predetermined levels. Humidity is instrumental to allow penetration of EtO in spores by softening their membrane.
Preconditioning may be required to bring large loads within predetermined humidity and temperature ranges. - Exposure: The targeted EtO concentration, humidity and temperature are stabilized until the end of the predetermined exposure time.
- Removal : EtO residues are withdrawn by vacuum pulses. During an aeration phase, time is given to EtO to desorb from the load. Aeration may be continued outside the chamber under strictly controlled occupational health and safety conditions. Total aeration time varies between 12 and 48 hours
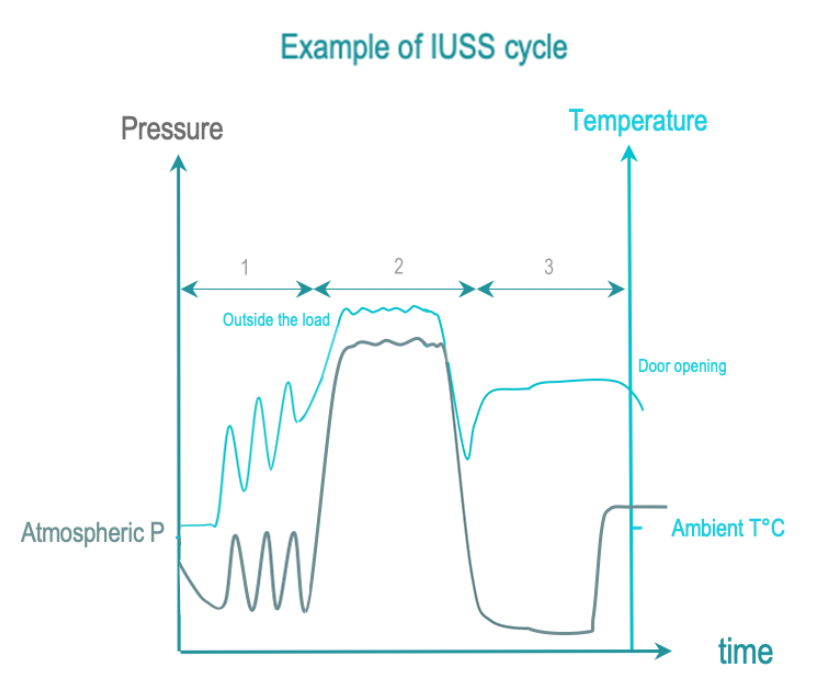
Immediate use steam sterilization (IUSS) is a variant of steam sterilization intended for emergency point-of-use non-terminal sterilization. RMD’s are not packaged. Transfer to user is immediate and performed with care in controlled environment. Compared to steam sterilization, RMD are not packaged, drying and cooling are usually shortened to limit cycle time. So, an RMD may hence still be wet on cycle completion, thereby increasing the risk of environmental contamination.
The 3 phases of IUSS are as follows :
- Conditionning: Vacuum and steam injection withdraw air from the chamber and load, and replace it with saturated steam. The load temperature is progressively increased.
- Exposure: The saturated steam injection is completed and given time to diffuse throughout the load.
- Removal : Condensation is withdrawn by vacuum and heating. The load is allowed to cool down after opening the door for safe handling by the operator.
Some countries do not allow IUSS, others may tolerate it. Recommendations usually advise to make an evaluation regarding the need for point-of-use reprocessing. RMD inventory may have to be improved to afford reprocessing by a central sterilization department.
![]() There are no international standards for IUSS.
There are no international standards for IUSS.
![]() Local regulations or guidelines may require or recommend that sterilization takes place in centralized sterilization department thereby prohibiting point of use sterilization. Operating theatres are usually not equipped and organized to clean and dry RMD’s as consistently as a centralized sterilization department.
Local regulations or guidelines may require or recommend that sterilization takes place in centralized sterilization department thereby prohibiting point of use sterilization. Operating theatres are usually not equipped and organized to clean and dry RMD’s as consistently as a centralized sterilization department.
Exposure of all RMD surfaces to a liquid sterilant for a controlled time, temperature and concentration yields a targeted SAL. Rinsing must preserve the SAL.
The most common sterilant is peracetic acid. Cleaning is usually separated from sterilant application.
Acceptance of the liquid sterilant concept depends on the region. In some countries, it may be accepted or tolerated as an alternative to high or low temperature sterilization for some fragile or heat sensitive RMD’s. In other countries, this practice may be considered as a deviation to Spaulding classification principles.
- Conditioning: Conditioning may not be required. Cleaning may be included in the cycle or take place separately.
- Exposure:All RMD surfaces are exposed to the sterilant at predetermined temperature and conditions and for predetermined time.
- Removal : Sterilant residues are eliminated by rinsing. Rinsing preserves the SAL. If drying is included in the cycle, drying preserves the SAL.
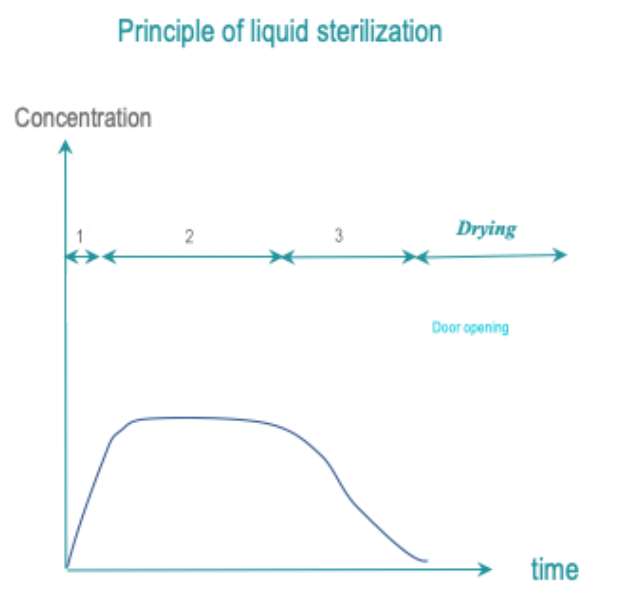
Written cleaning and disinfection standard operating procedures (SOP’s) are prepared according to quality management principles.
User supervises or performs and controls a process validation :
- Installation of Sterilizer is conform to manufacturer recommendations
- IFU, test and calibration certificates are available
- Standard Operating Procedures (SOP’s) are up to date. For newly purchased RMD, a new SOP is defined if an existing one cannot be used.
- Routine controls are in place
- Occupational health and safety measures are in place
- Training (including training on occupational health and safety measures) are up to date, executed and training certificates are available.
- Maintenance plans are in place for all sterilization equipments
- Traceability is operational

WFHSS key recommendations for sterilization
- Terminal Sterilization, compliant to international standards, is preferred to non-terminal sterilization. Steam at 134°C or 132°C is the preferred sterilization method and may be used when specified by an RMD manufacturer’s IFU. Exposure time vary according to country regulations or guidelines. Dry heat sterilization should be replaced by steam.
- For RMD’s that are not compatible with steam at 132°C or 134°C , an RMD manufacturer’s IFU indicates the sterilization methods between either steam at 121°C to 125°C or low temperature sterilization and cycles to be used. Choice of LTS methods is according to regulatory, guidelines, practical or occupational health and safety considerations.
Liquid sterilization claims remain to be evaluated. - Obtention of a sterile and safe device requires process validation’s are defined according to quality management principles and the IFU of RMD and sterilizer manufacturers.
IUSS to be replaced by steam sterilization
Go to IUSS sterilization →
1 of 16 Liquid sterilantTo be evaluated by WFHSS
Go to Liquid sterilization →
2 of 16 SteamSteam 134°C or 132°C preferred when allowed by RMD IFU
Go to Steam sterilization →
3 of 16 LTSFCycle according to RMD IFU
Go to Low temperature steam formaldehyde →
4 of 16 VH2O2Cycle according to RMD IFU
Go to Vaporized H2O2 →
5 of 16 EtOCycle according to RMD IFU
Go to Ethylene Oxide →
6 of 16 Clean, dry, packaged RMDNon packaged for non terminal sterilization
Go to choice of sterilization process →
7 of 16 Sterile Medical DeviceNon packaged RMD for immediate use when non terminal sterilization
Packaged RMD for storage when terminal sterilization
Go to choice of sterilization process →
Terminal sterilization preferred to
non terminal
Go to choice of sterilization process →
9 of 16 Non terminal sterilizationThe RMD is not protected by a packaging and must be immediately used after sterilization
Go to choice of sterilization process →
10 of 16 +Terminal sterilization preferred
Go to recommendation of WFHSS for sterilization →
11 of 16 +Steam sterilization at 132°C or 134°C preferred when allowed by RMD IFU
Go to recommendation of WFHSS for sterilization →
12 of 16 +Visual control and routine controls
Go to Sterilization and quality management →
13 of 16 +According to RMD IFU
Go to choice of sterilization process →
14 of 16 +Steam sterilization at 132°C or 134°C preferred when allowed by RMD IFU
Go to recommendation of WFHSS for sterilization →
15 of 16 +Visual control and routine controls
Go to Sterilization and quality management →
16 of 16- ISO 17665-1: Sterilization of health care products – Moist heat – Requirements for the development, validation and routine control of a sterilization process for medical devices – 2006
- ISO 17665-2: Sterilization of health care products – Moist heat – Part 2: Guidance on the application of ISO 17665-1 – 2009
- ISO 17665-3: Sterilization of health care products – Moist heat – Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization – 2013
- EN 285: Sterilization – Steam sterilizers – Large sterilizers – 2016
- EN 13060: Sterilization – Steam sterilizers – Small steam sterilizers – 2018
- ISO 20857: Sterilization of health care products – Dry heat – Requirements for the development, validation and routine control of a sterilization process for medical devices – 2014
- ISO 11135: Sterilization of health care products – Ethylene oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices – 2014
- EN 1422: Sterilizers for medical purposes – Ethylene oxide sterilizers – requirements and test methods – 2014
- ISO 25424: Sterilization of health care products – Low temperature steam and formaldehyde – Requirements for the development, validation and routine control of a sterilization process for medical devices – 2018
- ISO 14937: Sterilization of health care products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices – 2009
- EN 14180: Low temperature steam and formaldehyde sterilizers. Requirements and testing
- ISO 22441: Sterilization of health care products – Low temperature vaporized hydrogen peroxide – Requirements for the development, validation and routine control of a sterilization process for medical devices – (work in progress)
- EN 14180: Sterilizers for medical purposes – Low temperature hydrogen peroxide sterilizers – requirements and testing (work in progress)
- ISO 11140-1: Sterilization of healthcare products – Chemical indicators – Part 1: general requirements – 2014
- ISO 11140-3: Sterilization of healthcare products – Chemical indicators – Part 3: Class 2 indicator systems for use in the Bowie and Dick type steam penetration test – 2007
- ISO 11140-4 Sterilization of healthcare products – Chemical indicators – Part 4: Class 2 indicators as an alternative to the Bowie and Dick type test for detection of steam penetration – 2007
- ISO 11140-5 Sterilization of healthcare products – Chemical indicators – Part 5: Class 2 indicators for Bowie and Dick type air removal tests – 2007
- ISO 11140-6 Sterilization of healthcare products – Chemical indicators – Part 6: Class 2 indicators and process challenge devices for use in performance testing of steam sterilizers (work in progress)
- ISO 11138-1: Sterilization of health care products – Biological indicators – Part 1: General requirements – 2017
- ISO 11138-2: Sterilization of health care products – Biological indicators – Part 2: Biological indicators for ethylene oxide sterilization processes – 2017
- ISO 11138-3: Sterilization of health care products – Biological indicators – Part 3: Biological indicators for moist heat sterilization processes – 2006
- ISO 11138-4: Sterilization of health care products – Biological indicators – Part 4: Biological indicators for dry heat sterilization processes – 2017
- ISO 11138-5 : Sterilization of health care products – Biological indicators – Part 5: Biological indicators for low temperature steam and formaldehyde sterilization processes – 2017
- ISO/AWI 11138-6 : Sterilization of health care products – Biological indicators – Part 6: Biological indicators for hydrogen sterilization processes (work in progress)













