Medical device reprocessing is a complex combination of interrelated manual and automated tasks that must constantly adapt to surgical demand without compromising patient safety.
The Quality Management System (QMS) structures, documents device reprocessing activities to improve efficiency, meet applicable regulatory requirements while ensuring employee engagement and safety.
Quality Management System principles are defined by international standards. For the purpose of the present guide, ISO 134851 will serve as a reference.
![]() ISO 13485 is an adaptation for medical device domain, of the general purpose quality management standard ISO 9001. ISO 13485 is mainly intended for medical device manufacturing but also adapted to service activities such as device reprocessing by healthcare facilities. Several requirements specific to design and manufacturing of medical devices will however not be applicable. Abundant literature exists on ISO 13485 implementation in sterilization department, an example is AAMI ST 902.
ISO 13485 is an adaptation for medical device domain, of the general purpose quality management standard ISO 9001. ISO 13485 is mainly intended for medical device manufacturing but also adapted to service activities such as device reprocessing by healthcare facilities. Several requirements specific to design and manufacturing of medical devices will however not be applicable. Abundant literature exists on ISO 13485 implementation in sterilization department, an example is AAMI ST 902.
![]() ISO 13485 may be used to certify the QMS of an organization. Certification provides confidence to customers and external partners of a CSSD that the QMS is fully operational. It is attributed after audits by an external accredited consultant. Certification of the QMS according to ISO 13485, ISO 90013 or an alternative framework such as JCI4) requires support of consultant and resources that are not affordable for all sterilization departments.
ISO 13485 may be used to certify the QMS of an organization. Certification provides confidence to customers and external partners of a CSSD that the QMS is fully operational. It is attributed after audits by an external accredited consultant. Certification of the QMS according to ISO 13485, ISO 90013 or an alternative framework such as JCI4) requires support of consultant and resources that are not affordable for all sterilization departments.
ISO 13485 requirements are organized as follows:
- Quality Management system reminds QMS principles and describes documentation requirements from management directives to detailed tasks.
- Management responsibility outlines the role of top management in QMS implementation, improvement and employee engagement.
- Resources Management covers human and infrastructure/equipment needs.
- Product Realization, renamed Device Reprocessing for the purpose of this guide, provides guidance to structure and perform activities.
- Measurement, Analysis and Improvement describes measures to monitor and improve the quality of execution
The above 5 items are interrelated and equally important when implementing or modifying a QMS. This can be illustrated by the graph below.
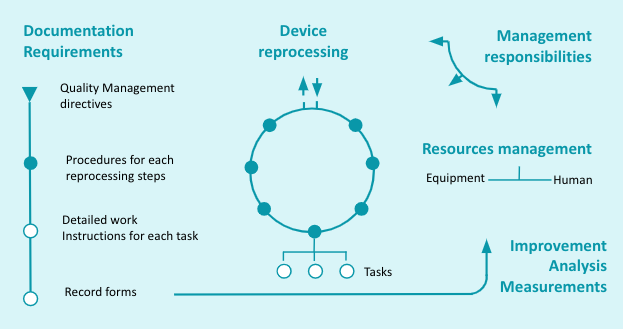 |
In the following paragraphs of this chapter we will review how each of these 5 parts of ISO 13485 apply to reprocessing of medical devices in Healthcare facilities.
The quality management system is described in a quality manual which
- States the purpose of the QMS and define quality objectives for each function of the sterilization department
- Identify and organize the various activities covered by the QMS and how these activities interact
- Define the various levels of documents used to execute and record activities
- Ensure that the documentation is updated and adequately used.
Management intentions are expressed in a quality policy statement. It is a synthetic document signed by Management. It expresses in concrete terms the engagement to respond to customers needs and improve the SMQ. The quality policy serves as a basis to establish the quality objectives assigned for each function of the sterilization department.
The Quality Policy and objectives are communicated and explained throughout the organization and reviewed for continuing suitability and applicability. (see also Management responsibility paragraph).
![]() An example of a “minimal” policy statement could be “our department is dedicated to the constant, on-time delivery of safe and fully operational devices to the surgical department in accordance with applicable regulation and best practices. This is achieved by precise execution of all steps of the device reprocessing path by skilled personnel and by their proactive contribution to the improvement of the Quality Management System”.
An example of a “minimal” policy statement could be “our department is dedicated to the constant, on-time delivery of safe and fully operational devices to the surgical department in accordance with applicable regulation and best practices. This is achieved by precise execution of all steps of the device reprocessing path by skilled personnel and by their proactive contribution to the improvement of the Quality Management System”.
A prerequisite to the implementation of the Quality Management System is a clear definition of the scope of the QMS, i.e. the processes (activities) covered by the QMS. Reprocessing of medical devices by health care facilities is typically described as a sequence of operational processes (point of use processing, cleaning, control & assembly, packaging, sterilization, transport and storage) execution of which require support processes (such as human resources, Management, Maintenance or qualification of equipment).
![]() The ISO definition for processes is “a set of interrelated or interacting activities that use inputs to deliver an intended result”. For example, the input to the cleaning process is a soiled device and the intended results is a clean and dry device (assuming that drying is part of the cleaning process).
The ISO definition for processes is “a set of interrelated or interacting activities that use inputs to deliver an intended result”. For example, the input to the cleaning process is a soiled device and the intended results is a clean and dry device (assuming that drying is part of the cleaning process).
![]() The scope of the sterilization department QMS may vary between institutions. For example, in some countries, flexible GI endoscope high level disinfection is placed under the responsibility of the sterilization department. Part of sterilization activities may also be subcontracted to an external organization. In that case, the healthcare facility must however check that the QMS of the external service provider is adequate.
The scope of the sterilization department QMS may vary between institutions. For example, in some countries, flexible GI endoscope high level disinfection is placed under the responsibility of the sterilization department. Part of sterilization activities may also be subcontracted to an external organization. In that case, the healthcare facility must however check that the QMS of the external service provider is adequate.
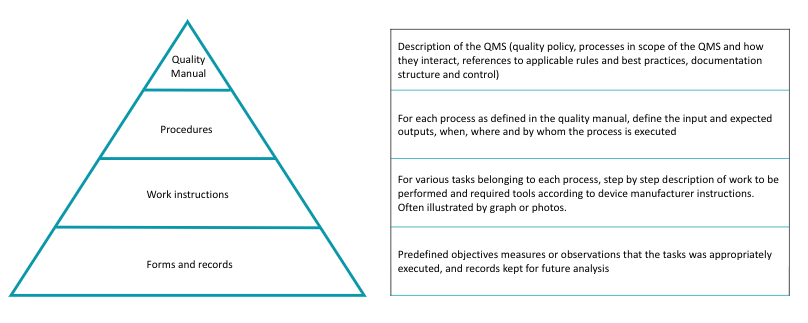
Once the processes are defined, procedures and related instructions are developed.
![]() Procedures relate to the process and define input, output, responsibilities, effectiveness criteria, measures or records and applicable laws and/or standards. Work instructions detail how to accomplish a specific job or task according to instructions for reprocessing provided by the Manufacturer of the Medical Device and best practices. For example the instructions related to a cleaning process will tell how to clean a specific device or family of devices that can share common cleaning guidance.
Procedures relate to the process and define input, output, responsibilities, effectiveness criteria, measures or records and applicable laws and/or standards. Work instructions detail how to accomplish a specific job or task according to instructions for reprocessing provided by the Manufacturer of the Medical Device and best practices. For example the instructions related to a cleaning process will tell how to clean a specific device or family of devices that can share common cleaning guidance.
A typical representation of documentation pyramid is displayed below.
Activities are monitored and recorded for traceability and future analysis. The data to be recorded, forms to be used and rules for conservation are defined by best practices and/or applicable local regulation.
A document control procedure is defined to ensure that QMS documentation is always up to date.
Procedures and instructions are developed according to a risk based approach. Risk is a combination of the probability of occurrence of harm and the severity of that harm. Consistent application of a proactive risk-based approach minimizes the risk of negative events and time consuming corrective measures.
The risk associated to each process may be summarized in a matrix that describes the risk and their consequences, rate their severity on a scale from 1 to 5. The 2 notes are multiplied to obtained a criticality grade which will determined the level of precaution to be taken. For example, a risk with minor consequences but very likely to occur might deserve more attention and investment than a risk with deadly consequences but that is unlikely.
It can be useful and educative to display the processes and their relationships on a map. Process mapping is specific to each sterilization department. An example is given below
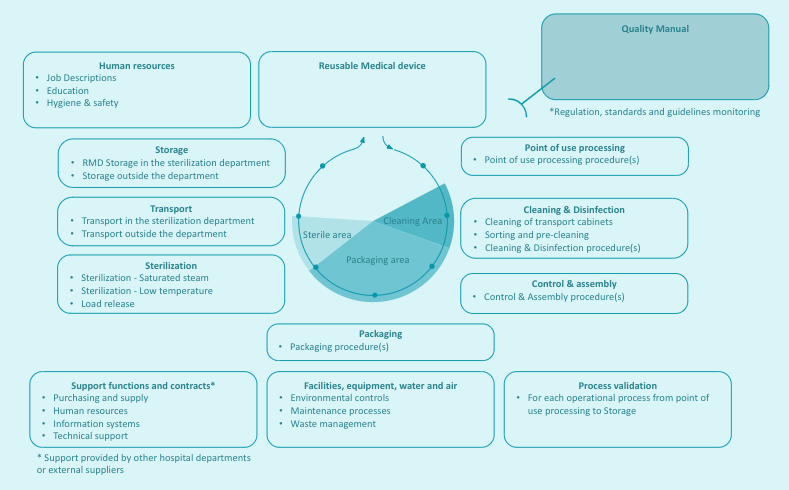 |
The implementation of a QMS is a strategic decision that must be actively supported by top management.
![]() Even if quality management is delegated to a quality Manager, management exemplarity is essential for engagement of staff and partners at all layers of the organization.
Even if quality management is delegated to a quality Manager, management exemplarity is essential for engagement of staff and partners at all layers of the organization.
Management:
- establishes quality policy and objectives (see Quality management system & documentation requirements),
- ensure that resources (human and equipments) are available (see Resources Management)
- defines, document and communicates responsibilities and authorities,
- organizes periodic Management reviews and seek internal and external feedbacks via audits to adapt to changes and improve performances. (see Measurement, Analysis and Improvement)
For ISO 13485, resources includes human resources and infrastructure, i.e. equipement as well as facilities, air and water
Needs are evaluated, defined and adapted by Management (see Management responsibilities), It includes
- Adequate staffing with appropriate skills and training. Competences are checked on scheduled basis and recorded. Personel is made aware of their contribution to quality.
- An adapted and safe work environment (light, T°C control, ergonomic workstation, protective equipement ) and clearly defined contamination control measures for each area of the sterilization department (air quality, dress code, circulation of devices and staff)
- Equipment with adequate performance and preventive maintenance plan
Reprocessing activities are organized according to user needs (for example composition of surgical sets, turnaround) and in accordance with applicable Regulation & Standard and best practices
Procedures for point of use processing, cleaning & Disinfection, Control & Assembly, Packaging, Sterilization, transport and storage are defined according to a risk based approach. Any change to procedure is evaluated before implementation.
Instructions are as detailed and illustrated as needed and in accordance with instructions for reprocessing provided by the Manufacturer of the Reusable Medical Device and Spaulding classification. Exception, must be justified.
When a new device is acquired or loaned it is determined if existing procedure and work instruction can be used or if adaptation or new instructions are needed.
Processes undergo Process Validation. Maintenance and Qualification of equipments are performed with calibrated measuring equipment
Human resources in charge of each reprocessing tasks are clearly identified, adequately trained (see resources management).
Manual and automated operation are monitored. Measures are benchmarked against pre-determined efficiency criteria. Records are kept. Device and surgical sets are traced.
![]() Traceability is the ability to verify the history, location, or application of a medical device by means of recorded identification. Identification of each surgical set, individual instrument can be obtained by bar code, direct marking on devices or color coding. Traceability allows the identification of loaned devices. It is used to determined the useful life of products.
Traceability is the ability to verify the history, location, or application of a medical device by means of recorded identification. Identification of each surgical set, individual instrument can be obtained by bar code, direct marking on devices or color coding. Traceability allows the identification of loaned devices. It is used to determined the useful life of products.
Procedures are defined to:
- Collect and analyze feedback from customers satisfaction, perform internal audit and management reviews, monitor processes via routine controls and process validations.
- Manage non conformities, collect and handle complaints, and when required by local regulation, report adverse event to health authorities
- Continuously maintain and improve the effectiveness of the Quality Systems with corrective and preventive measures.
The continuous improvement proinciple commonly illustrated by the Deming circle or PDCA (Plan – Do – Check – Act)
Deming is considered by many to be the father of modern quality control. Deming emphasized iteration of the scientific approach “hypothesis, experiment, evaluation” to continuously improve systems |
Validation of all steps of the sterilization processes from point of use processing to aseptic presentation is key to minimize the risk of a non sterile RMD
Go to WFHSS Recommandations for process validation →
1 of 5 QMS and documentation requirementsFor an example of validation of sterilization process
Go to Validation of steam sterilization →
For details on validations on packaging process
Go to Validation of packaging process →
3 of 5 Resource managementFor generale explanation on principles of validations
Go to Principles of validations →
4 of 5 Measurements, analysis, improvementFor details on validations on automated manual and ultrasonic assisted cleaning process
Go to Validation of cleaning process →
5 of 5
WFHSS recommendations for quality management
- Implementation of a Quality Management System is key to overcome the complexity of interacting sterilization activities, to meet applicable regulatory requirements and improve operational efficiency and safety.
- QMS requirements are defined by international standards. Although mainly intended for medical device manufacturing ISO 13485 guidance for documentation, management role, resources, operations and improvement apply to device reprocessing by healthcare facilities.
- The implementation and maintenance of a QMS relies on determination of management to make the quality policy statement a reality. Prerequisites are a clear definition of activities covered by the QMS (scope). Sterilization activities are commonly described as a sequence of sequential operations (from point of use cleaning to storage) and supporting functions (such as human resources, maintenance, purchasing). The quality manual identifies the applicable regulation and best practices and structures the activities and related documentation (procedure, instruction and reporting) and training according to a risk based approach. It describes how documentation, training are evaluated and improved and how non-conformance are treated. A realistic evaluation of resources that can be allocated to the maintenance of the QMS will determine if certification is affordable.
- ISO 13485 ISO 13485: Medical devices — Quality management systems — Requirements for regulatory purposes – 2016
- ANSI/AAMI ST90: Processing Of Health Care Products – Quality Management Systems For Processing In Health Care Facilities – 2017
- ISO 9001: Quality management systems — Requirements – 2015
- Joint Commission International http://www.jointcommissioninternational.org.













