The texts governing medical devices reprocessing and education activities may be grouped in 3 categories :
- Regulation (or legislation): Published by govermental bodies, they are of compulsory application. They can be national like in the USA1 or supra-national like in the European Union2. Example of key regulatory aspects pertaining to medical device reproccessing are the obligations for Medical Device Manufacturers to provide reprocessing instructions, for Healthcare facilities to report incidents related to Medical Device including those related to reprocessing. Regulation may also structure sterilization activities in a given country (for example, authorization to open a sterilization department, qualification required to Manage a CSSD, audit rules etc….), The present guidelines will not comment regulation.
- Standards (also referred to as norms): They set common expectations on products, services or processes to facilitate trade and enhance end user confidence and safety. Standards can be specific (for example steam sterilization processes or equipments) or transversal (for example, Quality Management). Standards are defined by consensus. Standards can be international (for example ISO), Regional (for example CEN for the European Union or national (DIN, BS etc..). National standards (i.e. applicable in only one country) will not be commented in this paragraph. ISO standards exist for sterilization but not for disinfection. Countries select the applicable standard for the various disinfection claim. (for example CEN and/or AOAC or ASTM)
- Standards are generally of voluntary application. Some can be made mandatory by local regulation. In practice, once a standard is adopted, it is convenient and safe for manufacturers, users and health care authorities to seek or verify compliance. When compliance is stipulated in a contract it is binding on the parties
- Guidelines (also referred to as recommended practices or best practices) are published by scientific societies, governmental bodies or standard organizations. Standards ease the interpretation of regulation and standards by healthcare facilities and serve as a framework for education programs and audits.
![]() At national level, guidelines are often co-written by 2 or more entities (for example Scientific societies and governmental bodies) or written by societies and endorsed by governments
At national level, guidelines are often co-written by 2 or more entities (for example Scientific societies and governmental bodies) or written by societies and endorsed by governments
In case of overlaps the applicable regulation always supersedes standards and guidelines.
ISO sterilization and washer-disinfector standards are under the responsibility of Technical Committee 198 (TC 198). EN sterilization and washer-disinfection standards are under CEN TC 102, and CEN TC 204.
Standards are prepared by experts nominated by their national delegations.
Proposal for a new standard may be submitted via a national delegation or by ISO or CEN technical committees. Once the scope of new work item proposal is approved by a majority of ISO or EN country members, the project is assigned by the Technical Committee to a working group. The first draft is often prepared by the initiators of the project. It is circulated for comments by national committees. Comments are then reviewed one by one by the working group experts. Several rounds of drafts, votes and comments review are usually needed until a consensual text is obtained and submitted for final approval. Along the road, collaborative tests may be needed to align on performance thresholds. Details can be obtained on ISO or CEN websites. Once published, Standards are periodically submitted for revision, amendment, confirmation or withdrawal. |
![]() In the case of the European community, the compliance to an harmonized standard (i.e. a standard endorsed by the CEE) indicates that the essential requirement of the medical device regulation are met. Standards are also used as a reference by notified body for CE marking.
In the case of the European community, the compliance to an harmonized standard (i.e. a standard endorsed by the CEE) indicates that the essential requirement of the medical device regulation are met. Standards are also used as a reference by notified body for CE marking.
![]() Standards are subject to copyright and fees. Revenues generated by the sales of standards are used to finance the standardization effort and organizations.
Standards are subject to copyright and fees. Revenues generated by the sales of standards are used to finance the standardization effort and organizations.
There are many standards but not all products or processes are covered by standards. For example there are ISO and EN standards for automated cleaning and disinfection processes but none for manual processes.
The figure below illustrates ISO and CE framework for sterilization activities. It is provided for illustration purpose only. This diagram is subject to constant change and only valid for the indicated period. Official list and status can be found on ISO and CEN websites
![]() Radiation sterilization standards which are not commonly used in routine in healthcare facilities are not represented.
Radiation sterilization standards which are not commonly used in routine in healthcare facilities are not represented.
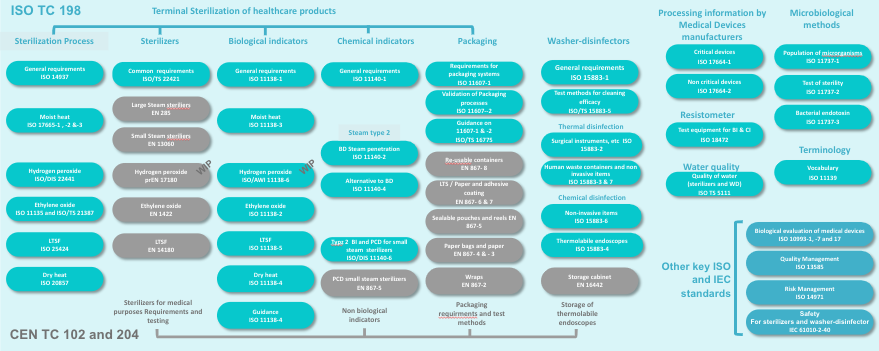 |  |
Some new standards in development (but not yet published) are displayed with a WIP tag (Work In Progress).
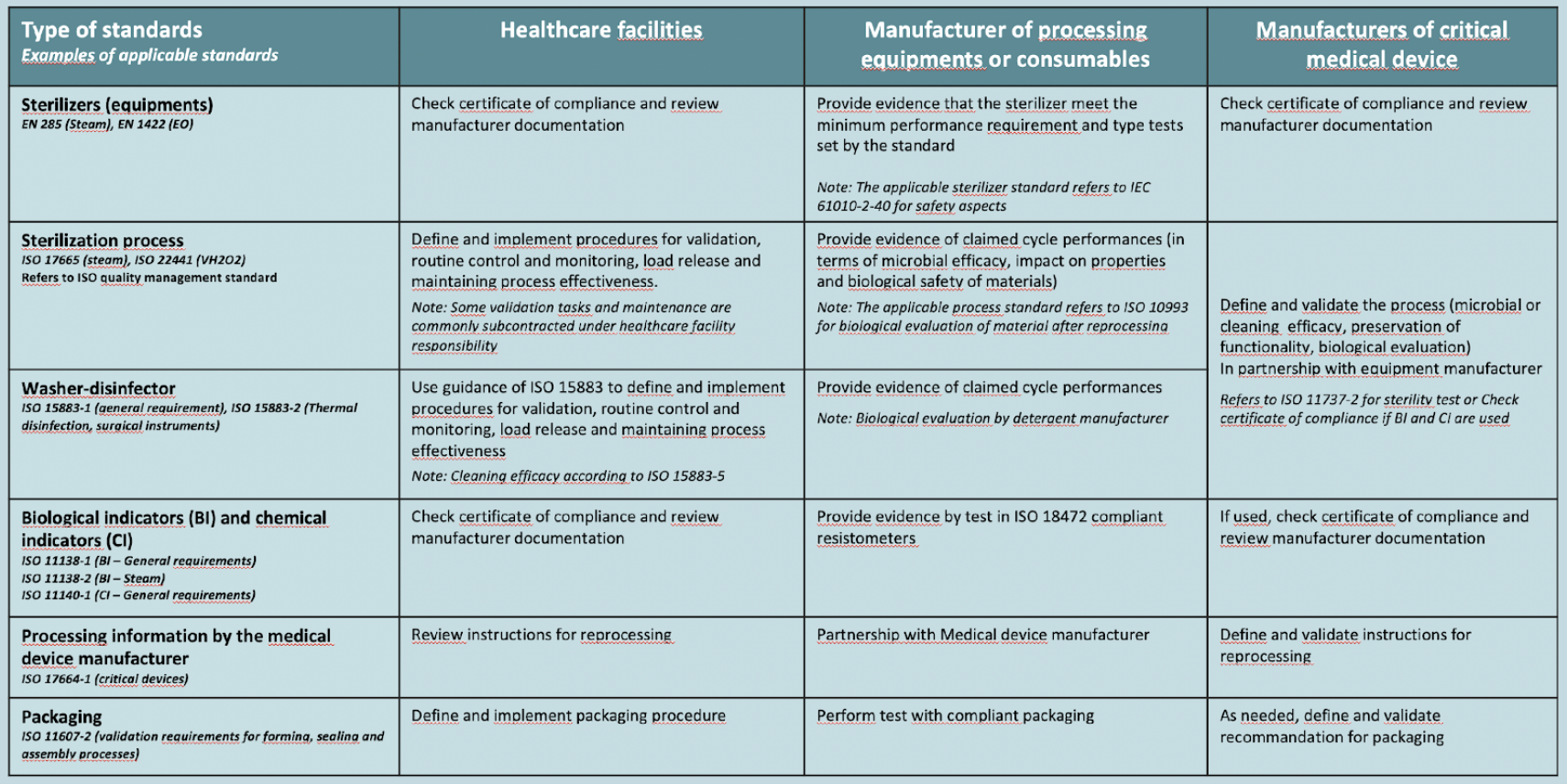
Sterilization process standards are intended for all stakeholders involved in device reprocessing i.e. healthcare facilities, manufacturers of reprocessing equipment or consumables, as well as manufacturers of medical devices and service providers.
For example, the sterilization process standards3,4,5,6,7,8,9,10 will be used by:
|
Packaging standards 11,12 and guidance 13 will be used by healthcare facilities for packaging process validation. Most of the requirements included in the standards will however be for manufacturers of single use devices and for manufacturer of sterile barrier systems (SBS) and for packaging materials.
Other standards or guidances are primarily intended for manufacturers of sterilizing equipment, consumables or medical devices. They define the requirements and test methods to be applied to justify compliance. Those standards are however also of interest for healthcare facilities. For example, sterilizers standards may be used to prepare tenders,
Examples of non TC 198 standards to which ISO and EN sterilization standards commonly refer are:
ISO 1099351,52,53 for the evaluation of the biological safety of the material after exposure to a sterilization or cleaning process |
ISO standards for thermal and chemical washer-disinfectors 35,36,37,38,39 are also primarily intended for manufacturers. However they contain guidance for healthcare facilities to check the conformity of installed washer-disinfector throughout its working life. ISO 15883-440 and EN 1644241 are dedicated to heat sensitive flexible endoscopes (reprocessing and storage respectively). ISO 15883-542 describes test method and criteria for cleaning efficacy
![]() EN sterilization standards are often referred to as equipment standards and ISO standards as process standards. This is not an official ISO language but it well depicts the respective role of each group of standards.
EN sterilization standards are often referred to as equipment standards and ISO standards as process standards. This is not an official ISO language but it well depicts the respective role of each group of standards.
Other ISO TC 198 standards and guidance displayed on the above diagram come in support of other TC 198 documents.
Examples of non TC 198 standards to which ISO and EN sterilization standards commonly refer are:
ISO 1099351,52,53 for the evaluation of the biological safety of the material after exposure to a sterilization or cleaning process |
In the above diagram key standards not belonging to ISO TC 198, EN TC 102 or TC 204 but to which ISO and EN standards refer, are also displayed
Examples of non TC 198 standards to which ISO and EN sterilization standards commonly refer are:
ISO 1099351,52,53 for the evaluation of the biological safety of the material after exposure to a sterilization or cleaning process |
The following diagram illustrates how the various sterilization, washer-disinfector, BI, CI, processing information and packaging standard may be used by healthcare facilities, manufacturers of processing equipments and consumables as well as medical device manufacturers
Normative language can sometimes be difficult to translate into practical considerations for healthcare facilities. Some possible explanations are listed below (not exhaustive):
- Standards contributes to the improvement of business practices by stimulating competition, innovation and avoid products that could put operator or patient at risk. Normative language hence is carefully chosen to avoid commercial bias, avoid design restrictive requirement which could hinder innovation, ensure complementarity with safety standards.
- Sterilization standards apply to reprocessing of medical device by both healthcare facilities and manufacturers of medical devices. Normative Requirements must hence be compatible with 2 domains with significant differencies.
![]() An example of a difference between healthcare facilities and device manufacturing industry are loads configurations: Typical single use medical device manufacturer loads are homogenous (i.e. same single use item with initial contamination levels under control. Typical healthcare facility load are heterogenous (i.e. different devices with variable levels of contamination even after thorough cleaning)
An example of a difference between healthcare facilities and device manufacturing industry are loads configurations: Typical single use medical device manufacturer loads are homogenous (i.e. same single use item with initial contamination levels under control. Typical healthcare facility load are heterogenous (i.e. different devices with variable levels of contamination even after thorough cleaning)
- Standard must be compatible with local guidelines or regulation. For example, responsibility assignments (who does what) are considered as guidelines or regulation matters are not addressed by standards.
![]() Some standards provide informative (non normative) guidance that will describe responsability assignements for various scenario such as healthcare facility.
Some standards provide informative (non normative) guidance that will describe responsability assignements for various scenario such as healthcare facility.
- Among the standards of highest level of interest to user there are the sterilization process standards. The table content of those standard is aligned on ISO 14937.
The sterilization process standards contain a foreword, an introduction,12 normative paragraphs and a variable number of normative or informative annexes.
The first three normative paragraphs equally apply to all categories of potential user of the standards.
|
Unlike for sterilization, there are no universal ISO Standards for evaluation of disinfection chemicals. Depending on the region different methods and acceptance criteria apply.
![]() For example EN standards in Europe, AOAC or ASTM in USA. Some countries may look at both EN and AOAC and ASTM, in some cases, with adaptations (for example Australia)
For example EN standards in Europe, AOAC or ASTM in USA. Some countries may look at both EN and AOAC and ASTM, in some cases, with adaptations (for example Australia)
Disinfectant are commonly classified in 3 categories: low level, intermediate level and high level. However the borders between each category are not universally defined. For example high level disinfection is always associated to some level of efficacy on spores but the efficacy threshold on spores is not the same.
An example of a commonly used definition for a sporicidal formulation is: a germicide that inactivates all microbial pathogens, except large numbers of bacterial endospores (Rutala 1990, Spaulding 1970). However the applicable test method and criteria is country dependent different. For example :
|
Unlike for sterilization in health care facilities, where sterility test are performed only with the germs predetermined as the most resistant, disinfection claims are for specific categories of germs : bacteria, fungi and yeast, virus (envelopped and non envelopped) and mycobacteria and spores, expressed in log reduction, with log reduction objective which may differ between catgories of microorganismes and region.
![]() For sterilization in healthcare facilities according to the overkill principle: > 6 log of the spore selected as the most resistant to the sterilization process and SAL of 10-6
For sterilization in healthcare facilities according to the overkill principle: > 6 log of the spore selected as the most resistant to the sterilization process and SAL of 10-6
In the European community the test method to be applied for the various type of disinfection claims are defined by EN 14885.
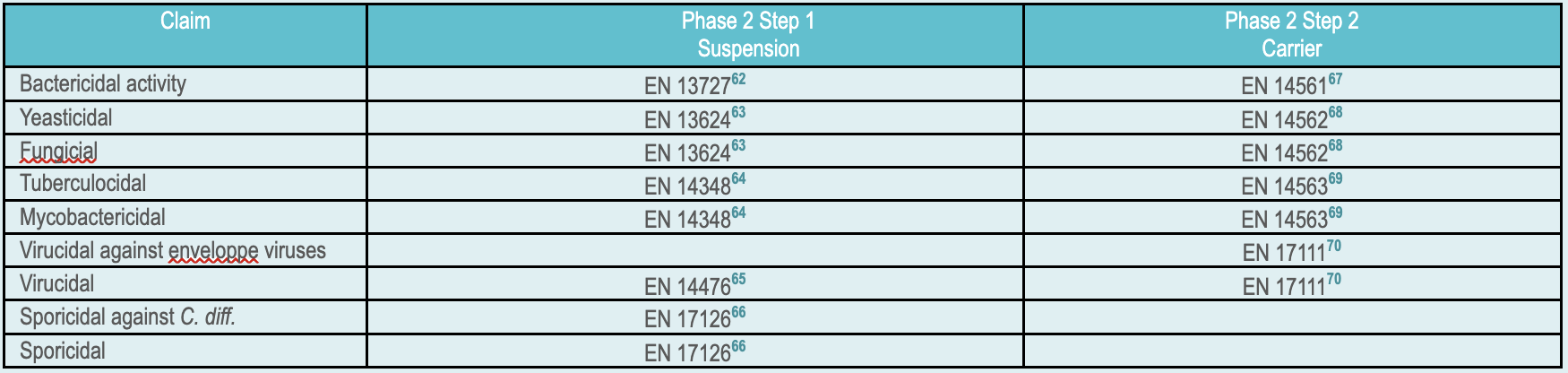
EN 1488561 covers human medicine, veterinary, food, industrial, domestic and institutional areas. Within the medical domain EN 14885 proposes adapted substantiating strategies for various field of application hand hygien (hygienic handrub, hygienic handwash, Surgical Handrub or-wash), surface disinfection (with or with mechanical action and airbone), textile disinfection and aqueous system and instrument disinfection. The rest of this paragraph will focus on disinfection of instruments. EN 14885 list test in suspension (phase 2.1) or on carrier (Phase 2.2). For medical devices, phase 2.2 carrier tests are more representative of real life and closer to AOAC and ASTM approach. Interference matter is added to simulates soil (in clean or dirty condition depending on the application) Test are performed at the concentration and exposure time specified by the manufacturer. At the end of the exposure time a neutralizer is added to stop the microbiocidal activity before counting to check that the targeted log reduction is achieved.
|
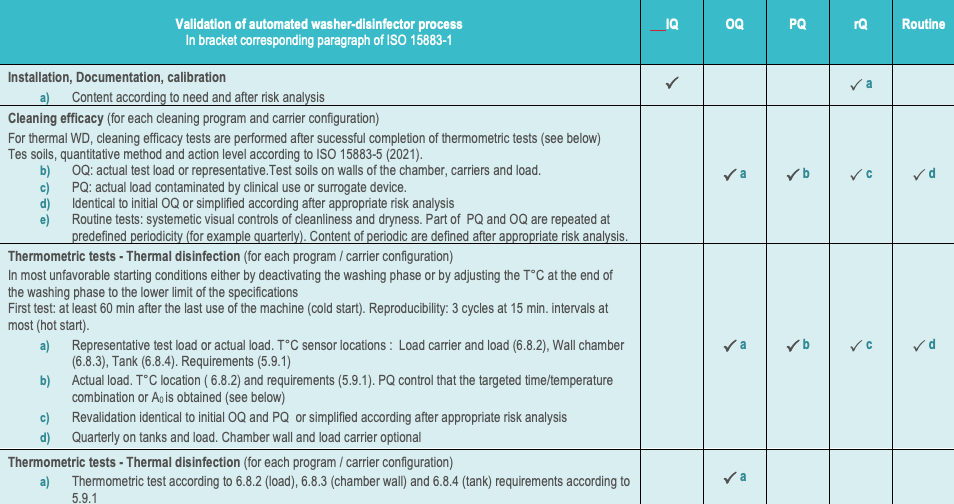
Unlike for disinfectant there are no international standard for the evaluation of cleaning efficacy but the efficacy of the cleaning process (i.e. combination of mechanical and chemical action) can be evaluated according to ISO 15883-5.
![]() Although ISO 15883-5 was developped for the evaluation of automated process. It may be applied to manual processes with the drawback, for manual processes, that there are operator dependence.
Although ISO 15883-5 was developped for the evaluation of automated process. It may be applied to manual processes with the drawback, for manual processes, that there are operator dependence.
The illustration below is an attempt to illustrate the objectives and related normative or regulation framework for terminal sterilization, liquid sterilant, the various categories of disinfectants, prion inactivation and detergent.
- The disinfection claims are according to efficacy on germs of increasing resistance (from envelopped viruses and bacteria to spores). Applicable tests methods and criteria are region or country dependent. High-level disinfectants must have some level of activity on the most resistant germs i.e. spores (In addition to being efficient on all other categories of micro-organisms). Test methods and thresholds for high-level disinfection are country/region dependent.
- Liquid sterilant is unique to US FDA (and country which follows FDA guidance). Sterilant means a 6 log reduction of spores. FDA reminds that liquid sterilant are not equivalent to sterilization.
- Exposure to a sterilant is followed by rinsing which may present a risk of recontamination and the liquide sterilized device is not protected by a sterile barrier system. Sterilant will hence only be used for critical devices when terminal sterilization cannot be performed.
- Terminal sterilization with steam or low temperature processes are the preferred methods for critical devices. Sterility is preserved until aseptic opening of the SBS.
- Prion inactivation is not universally standardized but recognized in vivo and in vitro methods exist. According to these tests methods, prion inactivation can be obtained by sterilization (for example some H2O2 cycles), some powerful detergent (for example high pH alkamine), or specific combination of detergent and sterilization processes. Some sterilization method are know as fixative on prion (i.e. reinforce their adherence on surfaces – for example dry heat, EO, Formaldehyde). Attention paid to prion risk is highly country dependent.
- There is no universal standard for evaluation of cleaning agent performances but cleaning process (i.e. mechanical action + Chemical action) can be benchmarked against ISO 15883-5.
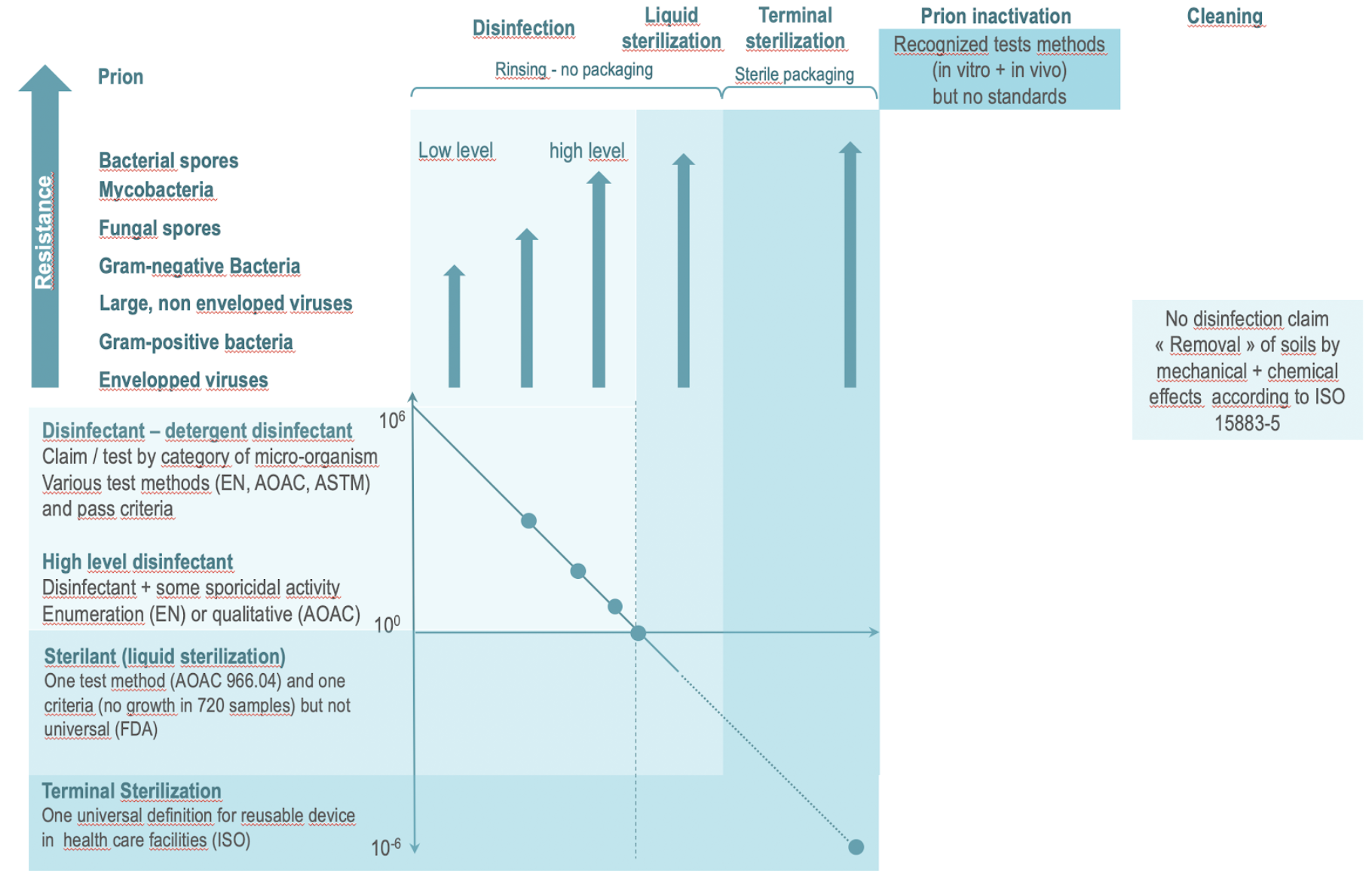 |
Guidelines may be specific to a given topic, for example steam sterilization or global like WFHSS guidelines. Some guidelines are access free like WFHSS. For other, fees must be paid.
WFHSS guidelines and other worldwide or regional guidance do not supersede local guidelines when they exist. See Guidelines for example of international, local or regional guidelines
- The specific objective of the WFHSS guidelines is to share and promote best practices and encourage alignment on best available evidence based practice.
It is often considered that significant differences exists between guidelines. In reality, core reprocessing directives are common and differences are in the how.
- For example, there is a consensus on need to avoid drying of soil on instrument but there can be different opinions on how this is best achieved. Another key difference which is often outined pertains to the use of biological indicators for load release. There again, difference may not be as big as it seems. All standards requires validation and systematic control of process parameters. In some countries however bacteriological indicators and chemical will be commonly used in routine while other will rely solely or mainly on careful revision of process parmeters (so-called parametric release). Countries which use BI often release the load before the BI can be read and use BI or CI as a double check.
- Sterilization training and practices are governed or guided by international and/or regulation, standards and guidelines. Core principles are common to all countries but interaction and role played by each category of text vary according to country history.
- Regulation is country or region specific. Some device reprocessing responsibilities are defined by national or supra-national medical device regulations. Organization of sterilization activities may also be defined by governmental bodies.
- International standards for sterilization and washer disinfector define common expectations that contribute to facilitate trade and enhance confidence. Applicable standardization framework may however vary according to countries. There are no ISO standards for disinfection and no universal definition of High Level Disinfection. Disinfectant activities evaluation method and acceptance criteria are defined by local regulation or guidance.
- Guidelines complete and ease interpretation of regulation and standards. They serve as a framework for education and trainings. WFHSS guidelines do not replace national guidelines when they exist but can be used when they are none or used to achieve progressive alignment on best practices across the world.
- Title 21 CFR parts 800-1299
- Regulation (EU) 2017/745 of the European Parliament and the Council of 5 April 2017 on medical devices
- ISO 17665-1: Sterilization of health care products – Moist heat – Requirements for the development, validation and routine control of a sterilization process for medical devices – 2006
- ISO 17665-2: Sterilization of health care products – Moist heat – Part 2: Guidance on the application of ISO 17665-1 (2009)
- ISO 17665-3: Sterilization of health care products – Moist heat – Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization (2013)
- ISO/DIS 22441: Sterilization of health carte products – Low temperature vaporized hydrogen peroxide – requirements for development, valudation and routine control of a steriization process for medical devices (Work In Progress)
- ISO 11135: Sterilization of health care products – Ethylene oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices (2014)
- ISO 25424: Sterilization of health care products – Low temperature steam and formaldehyde – requirements for development, valudation and routine control of a steriization process for medical devices (2018)
- ISO 20857: Sterilization of health care products – Dry heat – requirements for development, valudation and routine control of a steriization process for medical devices (2010)
- ISO 14937: Sterilization of health care products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices (2009)
- ISO 11607-1: Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (2019)
- ISO 11607-2: Packaging for terminally sterilized medical devices – Part 2: Validation requirements for forming, sealing and assembly processes (2019)
- ISO 16775: Packaging for terminally sterilized medical devices – Guidance on the application of ISO 11607-1 and ISO 11607-2 (2014)
- EN 285: Sterilization – Steam sterilizers – Large sterilizers – 2016
- EN 13060: Sterilization – Steam sterilizers – Small steam sterilizers – 2018
- EN 1422: Sterillizers for medical purposes – Ethylene oxide sterilizers – Requirements and test methods (2014)
- EN 14180: Sterilizers for medical purposes – Low temperature steam and formaldehyde sterilizers – requirements and testing (2014)
- EN 17180: Sterilizers for medical purposes – Low temperature vaporized hydrogen peroxide sterilizers – requirements and testing (Work In Progress)
- EN 868 2 to 10 Packaging for terminally sterilized medical devices – Part 2 to 10.
- ISO 11138-1 Sterilization of health care products — Biological indicators — Part 1: General requirements (2017)
- ISO 11138-2 Sterilization of health care products — Biological indicators — Part 2: Biological indicators for ethylene oxide sterilization processes (2017)
- ISO 11138-3 Sterilization of health care products — Biological indicators — Part 4: Biological indicators for moist heat sterilization processes (2017)
- ISO 11138-4 Sterilization of health care products — Biological indicators — Part 4: Biological indicators for dry heat sterilization processes (2017)
- ISO 11138-5 Sterilization of health care products — Biological indicators — Part 5: Biological indicators for low-temperature steam and formaldehyde sterilization processes (2017)
- ISO/AWI 11138-6 Sterilization of health care products — Biological indicators — Part 6: Biological indicators for hydrogen peroxide sterilization processes (Work In Progress)
- ISO 11138-7 Sterilization of health care products — Biological indicators — Part 7: guidance for the selection, use and interpretation of results (2019)
- ISO 11140-1: Sterilization of Health care products – Chemical indicators – Part 1: General requirements (2014)
- ISO 11140-3: Sterilization of Health care products – Chemical indicators – Part 3: Class 2 indicator systems for use in the Bowie and Dick-type steam penetration test (2007)
- ISO 11140-4: Sterilization of Health care products – Chemical indicators – Part 4: Class 2 indicators as an alternative to Bowie and Bowie and Dick-type test for detection of steam penetration test (2007)
- ISO 11140-5: Sterilization of Healthcare products – Chemical indicators – Part 5: Class 2 indicators for Bowie and Bowie and Dick-type air removal test (2007)
- ISO/DIS 11140-6: Sterilization of Healthcare products – Chemical indicators – Part 6: Type 2 indicators and process challenge devices for use in performance testing of small steam sterilizers Bowie and Bowie and Dick-type air removal test (Work In Progress)
- ISO 17664-1: Sterilization of health care products – Information to be provided by the medical device manufacturer for the processing of medical devices – Part 1 critical and semi critical medical devices (2021)
- ISO 17664-2: Sterilization of health care products – Information to be provided by the medical device manufacturer for the processing of medical devices – Part 2 non-critical medical devices (2021)
- ISO/DIS 22421: Sterilization of health care products — Common requirements for sterilizers for terminal sterilization of medical devices in health care facilities (2021)
- ISO 15883-1 : Washer-disinfectors — Part 1: General requirements, terms and definitions and tests (2006)
- ISO 15883-2 : Washer-disinfectors — Part 2: Requirements and tests for washer-disinfectors employing thermal disinfection for surgical instruments, anaesthetic equipment, bowls, dishes, receivers, utensils, glassware, etc. (2006)
- ISO 15883-3 : Washer-disinfectors — Part 3: Requirements and tests for washer-disinfectors employing thermal disinfection for human waste containers (2006)
- ISO 15883-6 : Washer-disinfectors — Part 6: Requirements and tests for washer-disinfectors employing thermal disinfection for non-invasive, non-critical medical devices and healthcare equipment (2011)
- ISO 15883-7: Washer-disinfectors — Part 7: Requirements and tests for washer-disinfectors employing chemical disinfection for non-invasive, non-critical thermolabile medical devices and healthcare equipment (2016)
- ISO 15883-4 : Washer-disinfectors — Part 4: Requirements and tests for washer-disinfectors employing chemical disinfection for thermolabile endoscopes (2018)
- EN 16442: Controlled environment storage cabinet for processed thermolabile endoscope (2015)
- ISO 15883-5: Washer-disinfectors — Part 5: Performance requirements and test method criteria for demonstrating cleaning efficacy (2021)
- ISO 11139: Sterilization of health care products – Vocabulary of terms used in sterilization and related equipment and process standards (2018)
- ISO 11737–1: Sterilization of health care products – Microbiological method – Part 1: Determination of a poluplation of microorganisms onproducts (2018)
- ISO 11737–2: Sterilization of health care products – Microbiological method – Part 2: Tests of sterility performed in the definition, validation and maintenance of a sterilization process (2018)
- ISO/CD 11737–3: Sterilization of health care products – Microbiological method – Part 3: bacterial endotoxin testing (Work In Progress)
- ISO/TS 5111: Quality of water for sterilizers, sterilization and washer-disinfectors (work in progress)
- ISO 13485: Medical devices — Quality management systems — Requirements for regulatory purposes (2016)
- ISO 14971: Medical devices – Application of risk management to medical devices (2019)
- IEC 61010-2-40: Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 2-40: Particular requirements for sterilizers and washer-disinfecfors used to treat medical material (2020)
- ISO 10993-1 Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process (2018)
- ISO 10993-7 Biological evaluation of medical devices – Part 7: Ethylene oxide sterilization residuals (2019)
- ISO 10993-17 Biological evaluation of medical devices – Part 7: Establishment of allowable limits for leachable substances (2002)
- AOAC 966.04-2002, Sporicidal Activity of Disinfectants
- AOAC 965.12-1967(2008), Tuberculocidal activity of disinfectants
- AOAC 955.17-1955, Fungicidal activity of disinfectants. Using trichophyton mentagrophytes
- AOAC 955.14-1959(2006), Testing disinfectants against salmonella
- AOAC 955.15-1959(2009), Testing disinfectants against staphylococcus aureus.
- AOAC 964.02-2012 Testing Disinfectants Against Pseudomoans aeruginosa
- ASTM E2197 Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals
- EN 14885 Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics (2022)
- EN 13727:2012+A2:2015: Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity in the medical area. Test method and requirements (phase 2, step 1)
- EN 13624:2021: Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of fungicidal or yeasticidal activity in the medical area – Test method and requirements (phase 2, step 1)
- EN 14348:2005: Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of mycobactericidal activity of chemical disinfectants in the medical area including instrument disinfectants. Test methods and requirements (phase 2, step 1)
- EN 14476 +A2 : Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of virucidal activity in the medical area – Test method and requirements (Phase 2/Step 1)
- EN 17126 EN 17126: 2018: Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectants in the medical area – Test method and requirements (phase 2, step 1)
- EN 14561:2006 : Chemical disinfectants and antiseptics. Quantitative carrier test for the evaluation of bactericidal activity for instruments used in the medical area. Test method and requirements (phase 2, step 2)
- EN 14562:2006: Chemical disinfectants and antiseptics – Quantitative carrier test for the evaluation of fungicidal or yeasticidal activity for instruments used in the medical area – Test method and requirements (phase 2, step 2)
- EN 14563:2008: Chemical disinfectants and antiseptics – Quantitative carrier test for the evaluation of mycobactericidal or tuberculocidal activity of chemical disinfectants used for instruments in the medical area – Test method and requirements (phase 2, step 2)
- EN 17111: 2018: Chemical disinfectants and antiseptics – Quantitative carrier test for the evaluation of virucidal activity for instruments used in the medical area – Test method and requirements (phase 2, step 2